Evangeline101
- 112
- 5
1. Homework Statement
2. Homework Equations
rate law equation
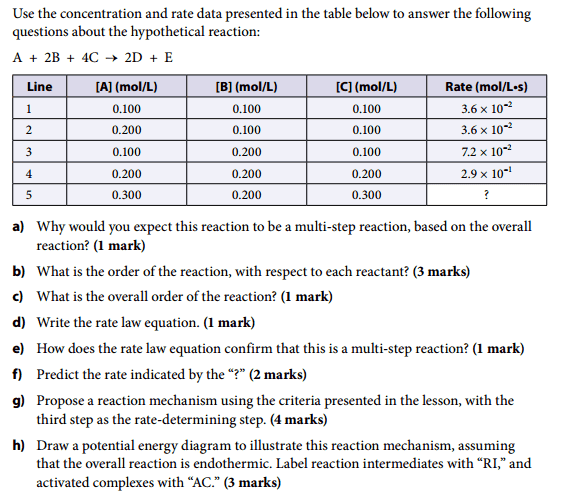
3. The Attempt at a Solution a) I would expect this reaction to be a multi-step reaction because there are six reactant molecules, as shown in the overall chemical equation. For this reaction to be one-step, all six molecules would have to collide at the same time, with proper orientation and sufficient energy. It is extremely unlikely that this would happen.
b)
i) Comparing lines 1 and 2: Doubling the [A] does not change the rate. The reaction is zero order, with respect to [A].
ii) Comparing lines 1 and 3: Doubling the (B) causes the rate to double. The reaction is first order, with respect to (B).
iii) Comparing lines 3 and 4: Doubling the [C] causes the rate to increase four times. The reaction is second order, with respect to [C].
c) Overall, it is a third-order reaction.
d) Rate law equation is: rate = k (B)[C]2
e) The rate law equation confirms that this is a multi-step reaction because the exponents of the rate law do not match the coefficients of the hypothetical chemical equation.
f) Comparing lines 1 and 5: The (B) has been doubled, so the rate should double (first order). The [C] has been tripled, so the rate should change by a factor of 9 (second order). The rate increases twice, and then nine times, for a total increase of eighteen times. The rate is 6.5 × 10-1 mol/L•s.
g) This is the part I am having trouble on. Can someone please explain how I should go about writing the reaction mechanism.
h) This part can be done once part g) is complete.Thanks.
2. Homework Equations
rate law equation
3. The Attempt at a Solution a) I would expect this reaction to be a multi-step reaction because there are six reactant molecules, as shown in the overall chemical equation. For this reaction to be one-step, all six molecules would have to collide at the same time, with proper orientation and sufficient energy. It is extremely unlikely that this would happen.
b)
i) Comparing lines 1 and 2: Doubling the [A] does not change the rate. The reaction is zero order, with respect to [A].
ii) Comparing lines 1 and 3: Doubling the (B) causes the rate to double. The reaction is first order, with respect to (B).
iii) Comparing lines 3 and 4: Doubling the [C] causes the rate to increase four times. The reaction is second order, with respect to [C].
c) Overall, it is a third-order reaction.
d) Rate law equation is: rate = k (B)[C]2
e) The rate law equation confirms that this is a multi-step reaction because the exponents of the rate law do not match the coefficients of the hypothetical chemical equation.
f) Comparing lines 1 and 5: The (B) has been doubled, so the rate should double (first order). The [C] has been tripled, so the rate should change by a factor of 9 (second order). The rate increases twice, and then nine times, for a total increase of eighteen times. The rate is 6.5 × 10-1 mol/L•s.
g) This is the part I am having trouble on. Can someone please explain how I should go about writing the reaction mechanism.
h) This part can be done once part g) is complete.Thanks.
Last edited: