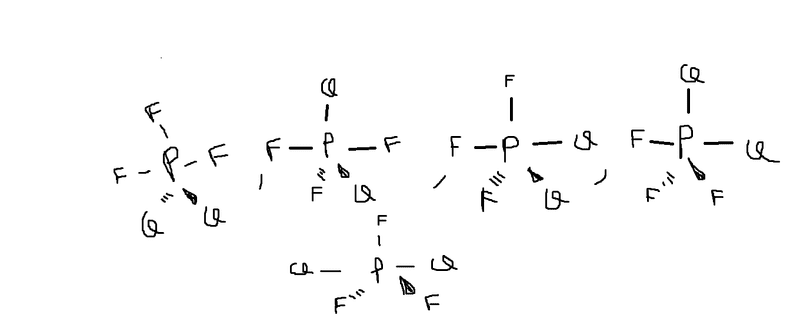
Discussion Overview
The discussion revolves around the number of stereoisomers for the compound PF3Cl2, focusing on the concepts of isomerism and mesocompounds. Participants explore their reasoning and calculations regarding the stereoisomer count.
Discussion Character
Main Points Raised
- One participant initially claims there are 5 stereoisomers but later acknowledges a potential mistake, suggesting the answer might be 3.
- Another participant proposes that certain structures are mesocompounds, which could affect the count of stereoisomers.
- Questions are raised about the differences between specific structures, indicating uncertainty in distinguishing between them.
- A later reply confirms the identification of mesocompounds and states they should be counted as stereoisomers.
- One participant admits to finding their mistake after further discussion.
Areas of Agreement / Disagreement
Participants express differing views on the number of stereoisomers, with some suggesting there are 3 while others initially counted 5. The discussion remains unresolved regarding the final count and the classification of certain structures as mesocompounds.
Contextual Notes
There are unresolved assumptions about the definitions of mesocompounds and how they influence the total count of stereoisomers. The discussion also reflects varying interpretations of the structures involved.