- #1
etotheipi
Consider the cell ##Pt | H_2 | H^+ || Ag^+ | Ag##. I’ve sketched the electric potential against distance across the cell:
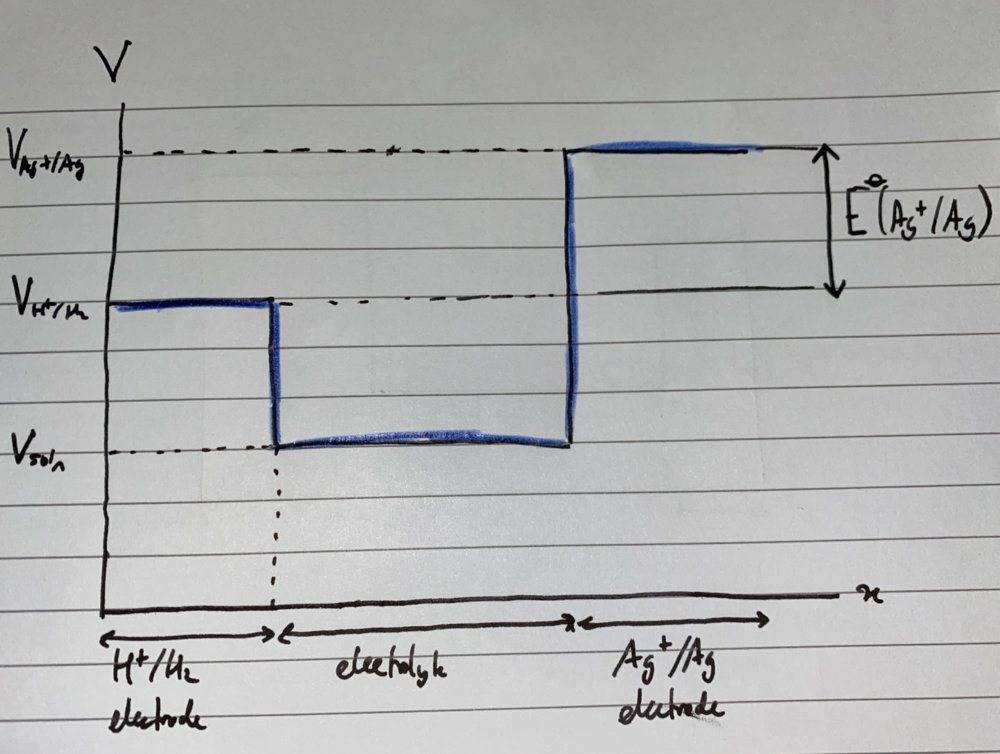
I would think the electrode potential of the ##Ag+/Ag## electrode is ##V_{Ag+/Ag} - V_{H^+/H_2}##, which I’ve labelled on the right. We set ##V_{H^+/H_2} = 0## as per convention.
However, my teacher states that the electrode potential equals the interfacial potential difference between the electrode and electrolyte, subtract (relative to) the interfacial potential difference of the reference electrode/electrolyte. This might well be true in this first example, but I don’t think it generalises so can’t be right!
For instance, consider now if we have a potential drop across the electrolyte (e.g. due to an electrolyte-electrolyte interfacial PD or a voltage drop of some other origin). The new potential diagram becomes,
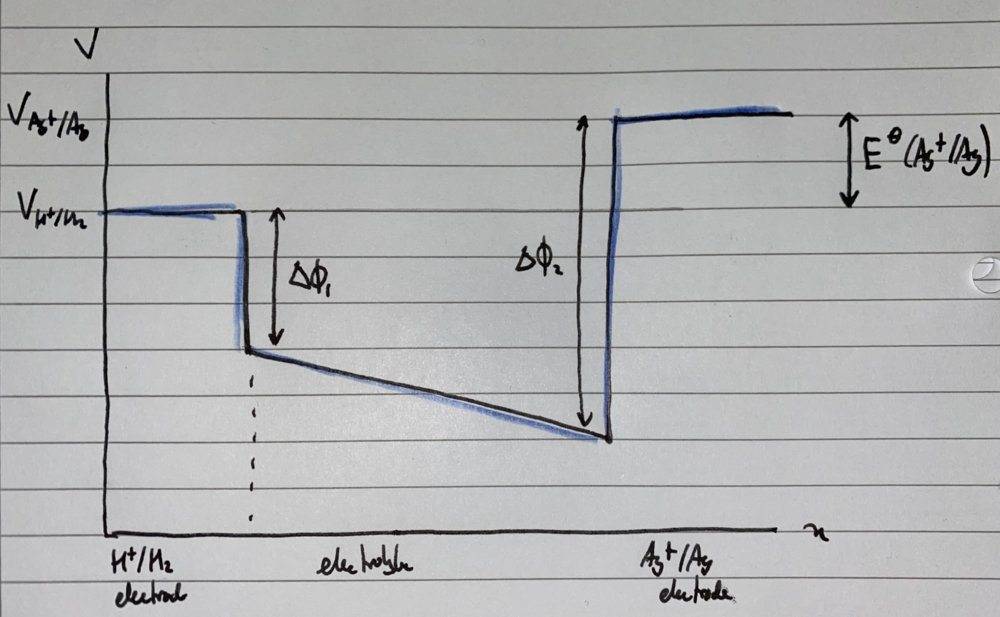
Ostensibly now, my teacher’s definition no longer gives the correct definition of standard electrode potential (the difference of the interfacial potential differences has changed!). Whilst I assume that##V_{Ag+/Ag} - V_{H^+/H_2}## is still the same as in the first example?
So I wondered whether someone could clarify as to which is correct?
I would think the electrode potential of the ##Ag+/Ag## electrode is ##V_{Ag+/Ag} - V_{H^+/H_2}##, which I’ve labelled on the right. We set ##V_{H^+/H_2} = 0## as per convention.
However, my teacher states that the electrode potential equals the interfacial potential difference between the electrode and electrolyte, subtract (relative to) the interfacial potential difference of the reference electrode/electrolyte. This might well be true in this first example, but I don’t think it generalises so can’t be right!
For instance, consider now if we have a potential drop across the electrolyte (e.g. due to an electrolyte-electrolyte interfacial PD or a voltage drop of some other origin). The new potential diagram becomes,
Ostensibly now, my teacher’s definition no longer gives the correct definition of standard electrode potential (the difference of the interfacial potential differences has changed!). Whilst I assume that##V_{Ag+/Ag} - V_{H^+/H_2}## is still the same as in the first example?
So I wondered whether someone could clarify as to which is correct?
Last edited by a moderator: