Discussion Overview
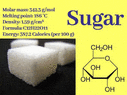
The discussion revolves around the molecular formula for sugar and the conventions for writing molecular formulas in general. Participants explore whether alternative arrangements of the molecular formula for sugar (C12H22O11) and sulfuric acid (H2SO4) are valid and discuss the rules governing these conventions.
Discussion Character
- Debate/contested
- Technical explanation
- Conceptual clarification
Main Points Raised
- One participant questions if the molecular formula for sugar can be rearranged into forms like H22C12O11 or O11C12H22, suggesting that there may be multiple valid representations.
- Another participant provides a similar example with sulfuric acid, proposing alternative arrangements of its formula, indicating a potential pattern in naming conventions.
- A participant clarifies that the molecular formula for saccharose is C12H22O11 by convention, emphasizing that this order is based on alphabetical arrangement and does not fully convey the molecular structure.
- There is a suggestion that the conventions for molecular formulas may not be as straightforward as alphabetical ordering, with references to the Hill and Richter systems for chemical formulas.
- A participant raises a question about the formula for ammonia (NH3), implying that it may not fit the discussed conventions.
- Another participant notes that historical reasons contribute to the established conventions for naming and writing molecular formulas, acknowledging that some names are well understood despite being unsystematic.
- One participant offers an opinion on the placement of cations and anions in chemical formulas, suggesting a general rule that may apply to both ionic and covalent compounds, while noting exceptions like ammonia.
Areas of Agreement / Disagreement
Participants do not reach a consensus on the validity of alternative molecular formula arrangements. There are multiple competing views regarding the conventions for writing molecular formulas, and the discussion remains unresolved.
Contextual Notes
The discussion highlights limitations in the understanding of molecular formula conventions, including the dependence on historical naming practices and the potential for ambiguity in different contexts.