Vishesh Jain
- 9
- 0
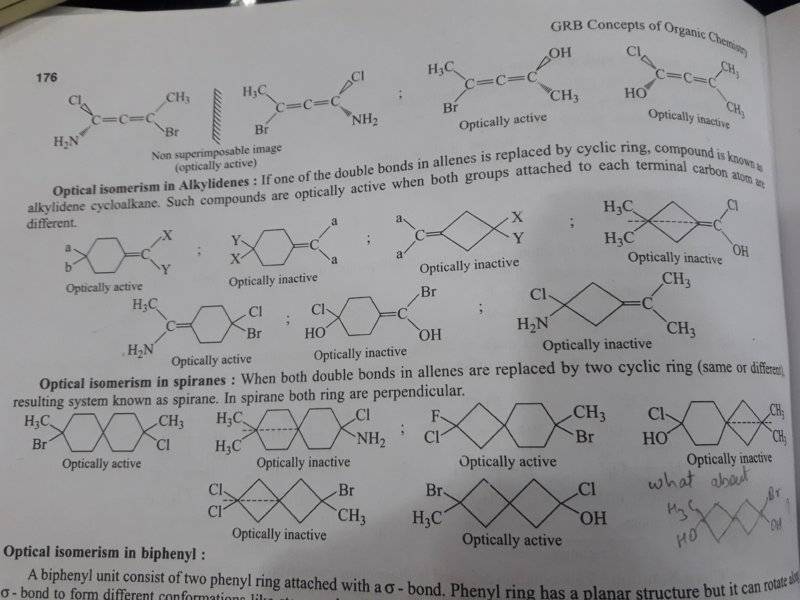
in the first image, under "optical isomerism in alkylidenes" , the middle compound in the second row is said as "optically inactive"
If the structure is as shown in https://chemistry.stackexchange.com/questions/58630/why-is-1-ethylidene-4-methylcyclohexane-chiral
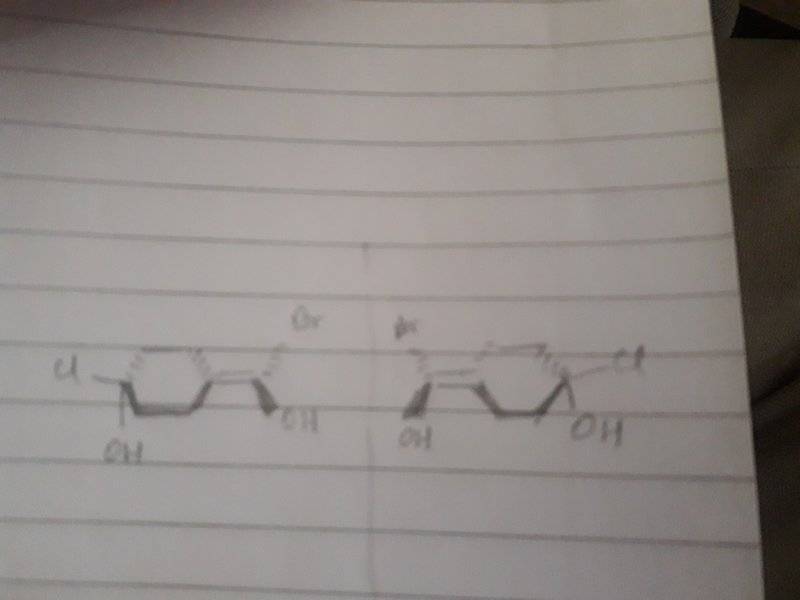
then it should be optically active (as i have drawn in the second image)
is the structure in the link correct ? is it an optically active compound ...? please explain in as much a detailed way as possible ... thanks ...
is 4-(bromohydroxy methenyl) 1 - chloro cyclohexan-1-ol a correct IUPAC name for it ?
If the structure is as shown in https://chemistry.stackexchange.com/questions/58630/why-is-1-ethylidene-4-methylcyclohexane-chiral
then it should be optically active (as i have drawn in the second image)
is the structure in the link correct ? is it an optically active compound ...? please explain in as much a detailed way as possible ... thanks ...
is 4-(bromohydroxy methenyl) 1 - chloro cyclohexan-1-ol a correct IUPAC name for it ?

