Janiceleong26
- 276
- 4
1. Homework Statement
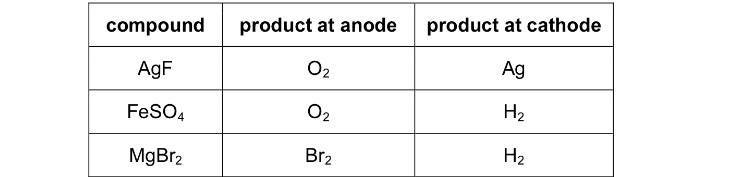
Why is the product at anode Br2 for MgBr2? Instead of O2?
 [/B]
[/B]
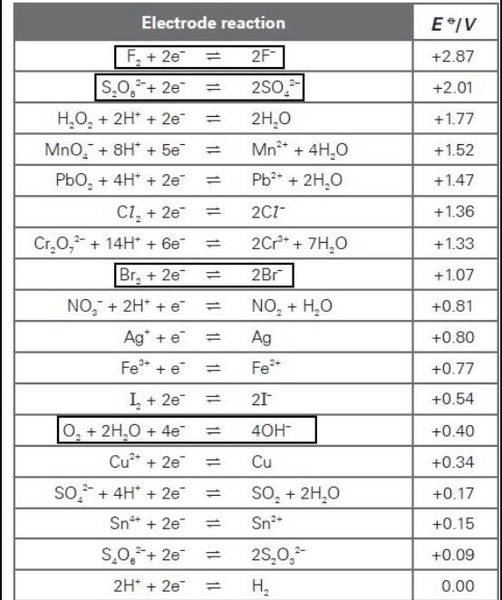
The EΘ value for the oxidation of OH- is -0.40 V,
And that of Br- is -1.07V
And so, shouldn't oxidation of OH- be easier? And hence, O2 is formed at the anode?
Why is the product at anode Br2 for MgBr2? Instead of O2?
Homework Equations
The Attempt at a Solution
The EΘ value for the oxidation of OH- is -0.40 V,
And that of Br- is -1.07V
And so, shouldn't oxidation of OH- be easier? And hence, O2 is formed at the anode?