- #1
alingy1
- 325
- 0
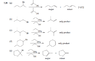
Please look at the image. For (b) and (c), what the solution manual says makes no sense to me.
You're reacting a primary alkyl halide with an unhindered base EtONa. They say that the only product is the alkene. Why do they not consider the SN2 reaction that WILL take place in larger quantity?
I'm uploading the question too.
You're reacting a primary alkyl halide with an unhindered base EtONa. They say that the only product is the alkene. Why do they not consider the SN2 reaction that WILL take place in larger quantity?
I'm uploading the question too.