duchuy
- 79
- 3
- Homework Statement
- Find the mechanism
- Relevant Equations
- x
Hi,
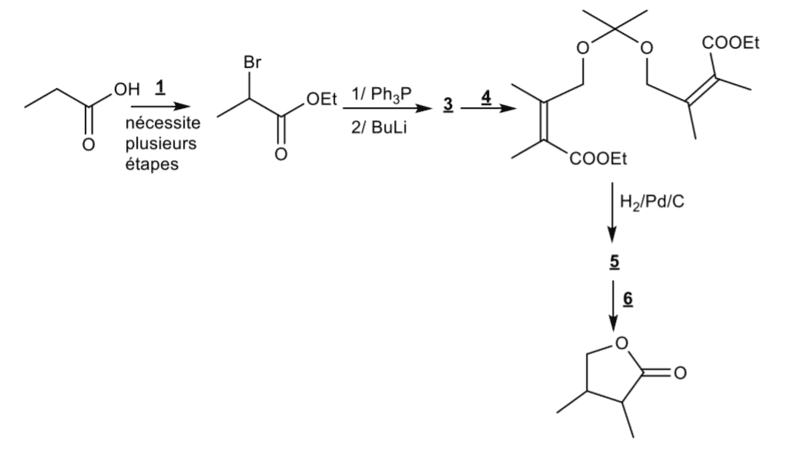
I'm trying to solve the number 5 and 6. Now I'm wondering what happens when I add H2/Pd/C on the 4th (after the question 4) molecule. I know that it will reduce pi bonds to sigma bonds, but will it affect the cetal group? Because if it does't I'm not quite sure that I'll be able to do the exercice with the steps given. My solution :
After adding Hd/Pd/C :
1) H+/H20, I will form 2 alcohol molecules (with the ester group) and acetone
2)Saponification : NaOH : Here I'm turning ester to carboxylate
3Intramolecular esterification : H+/H20 : I have to reacidify my environement in order to perform the esterification.
This would take 3 steps, while I only have step to do this (if H2/pd/C doesn't interact with the ketal group.
And if H2/Pd/C does give me my alcohol, it would also take me 2 extra steps to form my lactone.
Please help me find the solution for this.
By the way, what are the groups that H2 reduce?
I know that they reduce alcyne, alcene, cetone, aldehyde, ... the rest google is giving me mixed answers.
So what about : Nitrile, imine, carboxylic acid, ester, RCOCl, and acid anhydride?
Thank you so so much for your help!
I'm trying to solve the number 5 and 6. Now I'm wondering what happens when I add H2/Pd/C on the 4th (after the question 4) molecule. I know that it will reduce pi bonds to sigma bonds, but will it affect the cetal group? Because if it does't I'm not quite sure that I'll be able to do the exercice with the steps given. My solution :
After adding Hd/Pd/C :
1) H+/H20, I will form 2 alcohol molecules (with the ester group) and acetone
2)Saponification : NaOH : Here I'm turning ester to carboxylate
3Intramolecular esterification : H+/H20 : I have to reacidify my environement in order to perform the esterification.
This would take 3 steps, while I only have step to do this (if H2/pd/C doesn't interact with the ketal group.
And if H2/Pd/C does give me my alcohol, it would also take me 2 extra steps to form my lactone.
Please help me find the solution for this.
By the way, what are the groups that H2 reduce?
I know that they reduce alcyne, alcene, cetone, aldehyde, ... the rest google is giving me mixed answers.
So what about : Nitrile, imine, carboxylic acid, ester, RCOCl, and acid anhydride?
Thank you so so much for your help!