worryingchem
- 41
- 1
Hi, in class I'm doing a Suzuki Cross Coupling reaction based on
"Nickel-Catalyzed SuzukiMiyaura
Couplings in Green Solvents" by Stephen D. Ramgren, Liana Hie, Yuxuan Ye, and Neil K. Garg.
The reaction involved the bromo-aryl reacting with the boronic acid in heated t-amyl alcohol in the presence of NiCl2(PCy3)2 and tripotassium phosphate.
During the work-up, the first thing we did was transferred our solvent into a test tube of 1M HCl. The product is supposed to be in the aqueous layer, but I don't know how a biphenyl product would dissolve in the aqueous layer.
Can someone clear this up for me? Thank you.
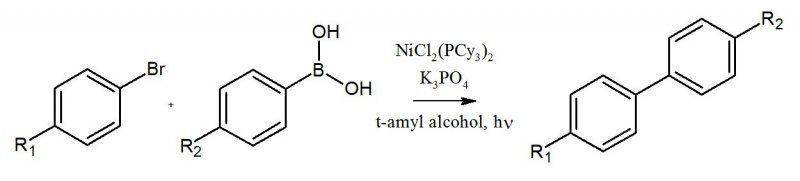
"Nickel-Catalyzed SuzukiMiyaura
Couplings in Green Solvents" by Stephen D. Ramgren, Liana Hie, Yuxuan Ye, and Neil K. Garg.
The reaction involved the bromo-aryl reacting with the boronic acid in heated t-amyl alcohol in the presence of NiCl2(PCy3)2 and tripotassium phosphate.
During the work-up, the first thing we did was transferred our solvent into a test tube of 1M HCl. The product is supposed to be in the aqueous layer, but I don't know how a biphenyl product would dissolve in the aqueous layer.
Can someone clear this up for me? Thank you.