PainterGuy
- 938
- 73
Hi,
I was reading about flame test and need your to understand few points.
Q1:
My question is about the part in red in the quoted text above. What does it mean when it says, "with hydrochloric acid to remove traces of previous analytes"?
Q2:
It also says, "The compound is usually made into a paste with concentrated hydrochloric acid, as metal halides, being volatile, give better results."
Sodium is a metal and sodium chloride is a metal halide. It's not volatile. Shouldn't the statement be qualified something like "...as metal halides, being GENERALLY volatile..."?
Q3:
The shown below is an electron configuration for sodium. In the compound sodium chloride the valence electron of sodium has been taken away by chlorine. In other words, sodium is left with its full 2p orbital. Is it electron(s) of 2p orbital which makes energetic transition to 3s orbital and then fall back to original 2p orbital and emit visible light photon?
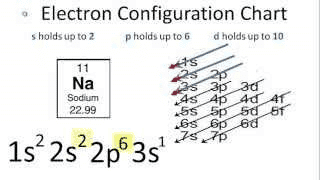
Source: https://terpconnect.umd.edu/~wbreslyn/chemistry/electron-configurations/configurationSodium.html
Helpful links:
1: https://www.quora.com/Does-pure-met...ts-I-know-metal-ions-do-how-about-pure-metals
2: https://www.quora.com/What-are-some-recommendations-for-a-chemistry-flame-test
3: https://chemistry.stackexchange.com/questions/75303/why-flame-color-of-salts-is-determined-by-metal
4: https://socratic.org/questions/why-are-chlorides-used-in-flame-test
I was reading about flame test and need your to understand few points.
Source: https://en.wikipedia.org/wiki/Flame_test#ProcessThe test involves introducing a sample of the element or compound to a hot, non-luminous flame, and observing the color of the flame that results. The idea of the test is that sample atoms evaporate and since they are hot, they emit light when being in flame. Bulk sample emits light too, but its light is not good for analysis. Bulk samples emit light with hydrochloric acid to remove traces of previous analytes.[1] The compound is usually made into a paste with concentrated hydrochloric acid, as metal halides, being volatile, give better results. Different flames should be tried to avoid wrong data due to "contaminated" flames, or occasionally to verify the accuracy of the color. In high-school chemistry courses, wooden splints are sometimes used, mostly because solutions can be dried onto them, and they are inexpensive. Nichrome wire is also sometimes used.[1] When using a splint, one must be careful to wave the splint through the flame rather than holding it in the flame for extended periods, to avoid setting the splint itself on fire.
Q1:
My question is about the part in red in the quoted text above. What does it mean when it says, "with hydrochloric acid to remove traces of previous analytes"?
Q2:
It also says, "The compound is usually made into a paste with concentrated hydrochloric acid, as metal halides, being volatile, give better results."
Sodium is a metal and sodium chloride is a metal halide. It's not volatile. Shouldn't the statement be qualified something like "...as metal halides, being GENERALLY volatile..."?
Q3:
The shown below is an electron configuration for sodium. In the compound sodium chloride the valence electron of sodium has been taken away by chlorine. In other words, sodium is left with its full 2p orbital. Is it electron(s) of 2p orbital which makes energetic transition to 3s orbital and then fall back to original 2p orbital and emit visible light photon?
Source: https://terpconnect.umd.edu/~wbreslyn/chemistry/electron-configurations/configurationSodium.html
Helpful links:
1: https://www.quora.com/Does-pure-met...ts-I-know-metal-ions-do-how-about-pure-metals
2: https://www.quora.com/What-are-some-recommendations-for-a-chemistry-flame-test
3: https://chemistry.stackexchange.com/questions/75303/why-flame-color-of-salts-is-determined-by-metal
4: https://socratic.org/questions/why-are-chlorides-used-in-flame-test