- #1
rwooduk
- 762
- 59
I understand that there are many attempts from inside the nucleus to tunnel out therefore if it was just one alpha particle trying to get in it wouldn't happen. I'm struggling with this derivation from class to describe the number of attempts:
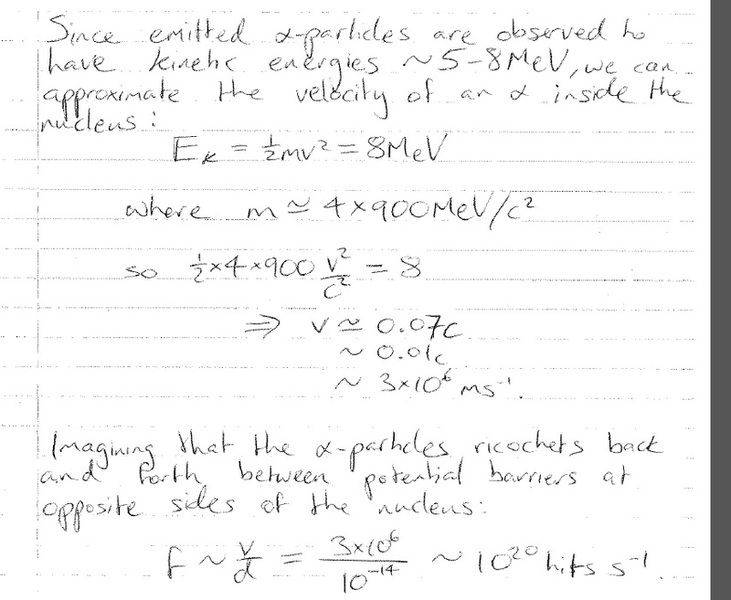
How does a velocity and size of the nucleus result in the number of hits per second? It works with dimensional analysis i.e. m/s / m = 1/s ---> frequency.
But this hits per second has been derived from a single alpha particle. I'm a little confused. Any insights on this rough derivation and an explanation would really be appreciated.
How does a velocity and size of the nucleus result in the number of hits per second? It works with dimensional analysis i.e. m/s / m = 1/s ---> frequency.
But this hits per second has been derived from a single alpha particle. I'm a little confused. Any insights on this rough derivation and an explanation would really be appreciated.