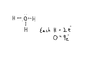SUMMARY
The discussion clarifies the charge states of the hydronium ion (H3O+) and the isocyanate ion (NCO-). H3O+ is positively charged due to the presence of three hydrogen atoms sharing electrons with oxygen, which has six valence electrons and forms covalent bonds, resulting in a stable octet configuration. Conversely, NCO- is negatively charged because it is short of one electron, leading to a formal charge of -1. The octet rule is referenced as a guideline for understanding electron configurations and stability.
PREREQUISITES
- Understanding of Lewis dot structures
- Familiarity with the octet rule in chemistry
- Basic knowledge of ionic and covalent bonding
- Concept of formal charge calculation
NEXT STEPS
- Study the formation and properties of hydronium ions (H3O+)
- Learn about the resonance structures of isocyanate (NCO-)
- Explore advanced concepts in electron counting and formal charge determination
- Investigate the limitations and applications of the octet rule in molecular stability
USEFUL FOR
Chemistry students, educators, and professionals seeking to deepen their understanding of molecular charge states and electron configurations in chemical compounds.