Recent content by stockzahn
-
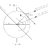
Graduate Linearization of nonlinear grad(T) in the diffusion equation
Dear all, I would like to perform numerical simulations of the heat transfer/temperature field in a static bath of superfluid helium. The heat conduction in superfluid helium can be modeled in two regimes depending on the heat flux. For low heat fluxes ##\dot{q}##, the temperature gradient...- stockzahn
- Thread
- Diffusion Diffusion equation Linearization Nonlinear
- Replies: 1
- Forum: Differential Equations
-

Question about Phase Change & Latent Heat
The specific evaporation enthalpy ##r## tells you how much heat has to be put into a fluid to evaporate 1 kg of it. The more heat ##Q## you have to cool away, the more mass ##m## has to be evaporated. Better to understand it entirely. If you have more questions, just ask.- stockzahn
- Post #14
- Forum: Introductory Physics Homework Help
-

Question about Phase Change & Latent Heat
Of course. Obvious, isn't it?- stockzahn
- Post #12
- Forum: Introductory Physics Homework Help
-

Question about Phase Change & Latent Heat
Then let's take your equation for the sensible heat transfer: ##Q = mc\Delta T##. As you've already stated yourself, for a latent heat transfer (evaporation), this equation changes to: ##Q = mr##. You know the heat and the evaporation enthalpy, therefore ##m=\frac{Q}{r}##. It says, the larger...- stockzahn
- Post #10
- Forum: Introductory Physics Homework Help
-

Question about Phase Change & Latent Heat
Well, then you can see the difference, one of the properties has the Temperature below the fraction bar, the other not. Suppose you have a coolant extracting a certain amount of heat from an object, e.g. 1 MJ. Does it matter what's the mass of the cooled object if you already know the heat you...- stockzahn
- Post #8
- Forum: Introductory Physics Homework Help
-

Question about Phase Change & Latent Heat
Azeotropic fluids are "pure" fluids. If they are vaporised, the temperature does not increase (given a constant pressure, which can be assumend when sweating). I just wanted to point out that a temperature difference isn't necessary (or even possible) in your scenario, so you have to find...- stockzahn
- Post #5
- Forum: Introductory Physics Homework Help
-

Question about Phase Change & Latent Heat
Ahoihoi @PF! - Water (sweat) is an azeotropic fluid. What does that mean for the temperature during evaporation? - The mass ##M## doesn't correspond to the mass of the human body - assuming a sensible heat transfer, what would ##M## stand for?- stockzahn
- Post #2
- Forum: Introductory Physics Homework Help
-

Balance reading when new object immersed in water
Right, 100 N. If floating, then mass of displaced water = mass of floating object, hence their weight (force) is identical.- stockzahn
- Post #20
- Forum: Introductory Physics Homework Help
-

Undergrad Why doesn't the solution to the Monty Hall problem make sense?
It's: You have three choices of equal probability (paths). Two of the three paths lead to the car, if you change. The third path leads to the car, if you don't change. Therefore in two out of three choices, changing is the winning strategy. Because you made your first door choice before the...- stockzahn
- Post #20
- Forum: Set Theory, Logic, Probability, Statistics
-

Undergrad Why doesn't the solution to the Monty Hall problem make sense?
You are right. Then differently: Why don't you accept the decision tree as mathematical method?- stockzahn
- Post #17
- Forum: Set Theory, Logic, Probability, Statistics
-

Undergrad Why doesn't the solution to the Monty Hall problem make sense?
What's mathematically wrong with decision trees?- stockzahn
- Post #14
- Forum: Set Theory, Logic, Probability, Statistics
-

Undergrad Why doesn't the solution to the Monty Hall problem make sense?
It's that: The original problem is based on the assumption that you have to choose one door before the host opens an empty one. But I just can agree with @PeroK. Take three cards and play the game hundred times - you are done within one hour. EDIT: Following these possible paths seems to me as...- stockzahn
- Post #11
- Forum: Set Theory, Logic, Probability, Statistics
-

Undergrad Why doesn't the solution to the Monty Hall problem make sense?
In the scenario you described you can start the game with just two doors left, since the host opens one empty door before you even chose one. He could have done that before you enter the room and it wouldn't change anything, so in your described scenario the chance of getting the car should be...- stockzahn
- Post #7
- Forum: Set Theory, Logic, Probability, Statistics
-

Balance reading when new object immersed in water
Then no. If the object's weight force is 100 N (##=m\cdot g##) and has a lower density, then it floats and therefore will displace water mass corresponding to the mass of the floating object (##m_{object} = m_{water} ## for ##\rho_{object} \leq \rho_{water} ##). If the volume of the displaced...- stockzahn
- Post #18
- Forum: Introductory Physics Homework Help
-

Balance reading when new object immersed in water
Independent of the density: If an object is immersed entirely it displaces an amount of water corresponding to its volume. So your statement is correct. Of course, an object displaces an amount of water corresponding to its (partially) immersed volume. Since it doesn't sink to the ground: -...- stockzahn
- Post #16
- Forum: Introductory Physics Homework Help