Lo.Lee.Ta.
- 217
- 0
"Remember that the alkyne must be a terminal alkyne and the halide must be primary. More than one combination of terminal alkyne and halide may be possible."
-This is what it says in the solutions manual of my Ochem book.
CH3CH2CH2C(triple bond)CCH3.
So the answer is CH3CH2CH2C(triple bond)C with 1. NaNH2 and 2. CH3Br
~OR~ HC(triple bond)CCH3 with 1. NaNH2 and 2. CH3CH2CH2Br
But I thought they said only primary halides can be used...?
Encyclopedia Britannica defines an alkyl halide as this: "In a primary alkyl halide, the carbon that bears the halogen is directly bonded to one other carbon, in a secondary alkyl halide to two, and in a tertiary alkyl halide to three."
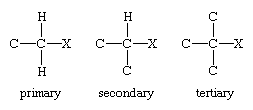
But how come we used a secondary halide in the 2nd possible reaction?
CH3CH2CH2Br is a secondary halide, right?
In the second practice problem, it asks how we can form (CH3)2CHC(triple bond)CCH2CH3
So the answer is only one possible reaction: (CH3)2CHC(triple bond)CH with 1. NaNH2 and 2. CH3CH2Br
But why can't we also have another possible reaction using HC(triple bond)CCH2CH3 with
1. NaNH2 and 2. (CH3)2CHBr ?
Thanks so much for your help! :)
-This is what it says in the solutions manual of my Ochem book.
In the first practice problem, they asked what you'd add to form CH3CH2CH2C(triple bond)CCH3.
So the answer is CH3CH2CH2C(triple bond)C with 1. NaNH2 and 2. CH3Br
~OR~ HC(triple bond)CCH3 with 1. NaNH2 and 2. CH3CH2CH2Br
But I thought they said only primary halides can be used...?
Encyclopedia Britannica defines an alkyl halide as this: "In a primary alkyl halide, the carbon that bears the halogen is directly bonded to one other carbon, in a secondary alkyl halide to two, and in a tertiary alkyl halide to three."
But how come we used a secondary halide in the 2nd possible reaction?
CH3CH2CH2Br is a secondary halide, right?
In the second practice problem, it asks how we can form (CH3)2CHC(triple bond)CCH2CH3
So the answer is only one possible reaction: (CH3)2CHC(triple bond)CH with 1. NaNH2 and 2. CH3CH2Br
But why can't we also have another possible reaction using HC(triple bond)CCH2CH3 with
1. NaNH2 and 2. (CH3)2CHBr ?
Thanks so much for your help! :)