Discussion Overview
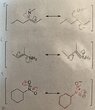
The discussion revolves around the correctness of answers related to resonance structures in organic chemistry. Participants explore the effects of inductive and mesomeric influences on electron distribution and resonance, addressing specific questions and examples provided by the original poster.
Discussion Character
- Debate/contested
- Technical explanation
- Conceptual clarification
Main Points Raised
- The original poster questions the correctness of their answers regarding resonance structures and discusses the inductive and mesomeric effects on electron behavior.
- One participant asserts that the original poster's understanding of the mesomeric effect is incorrect, specifically regarding the role of -OR as an electron donating group.
- The original poster expresses uncertainty about the correctness of their answers, particularly for the eighth question.
- Another participant clarifies that all resonance structures involve mesomeric effects, even when a pi bond is conjugated with a lone pair, and points out specific errors in the original poster's reasoning.
- There is a discussion about the transfer of pi bond electrons, with emphasis on the limitation of transferring electrons to saturated carbons.
- One participant highlights the importance of maintaining charge balance in resonance structures, specifically mentioning a positive charge on nitrogen in one of the examples.
Areas of Agreement / Disagreement
Participants express differing views on the correctness of specific resonance structures, with no consensus reached on all answers. Some participants agree on certain structures being correct, while others challenge the original poster's interpretations and reasoning.
Contextual Notes
Participants reference specific resonance structures and their characteristics, but there are unresolved aspects regarding the application of mesomeric and inductive effects. The discussion involves assumptions about charge conservation and the nature of electron movement in resonance.
Who May Find This Useful
Students and individuals studying organic chemistry, particularly those interested in resonance structures and electron effects in molecular systems.