Navin
- 112
- 34
- TL;DR
- Can We represent Isocyanides (RNC) with a dative bond ?
Okay guys, i had a question. Now in many indian publications and lecture rooms they say that we can represent HNC and isocyanides with a dative(coordinate) bond, but when i looked it up online, almost no one used such a structure. Wikipedia, Quora, Chem libre texts, etc, they describe it having a triple bond with charges.
Uh is the dative structure even correct ? Or is it an error that has been persistent is a bunch of indian publications ?Sources
With Dative Bond
1)Mtg-Interactive Irganic Chemistry by Girijesh Dubey (pg 623)
2)Resonance KVPY-SX Stream : Chemistry
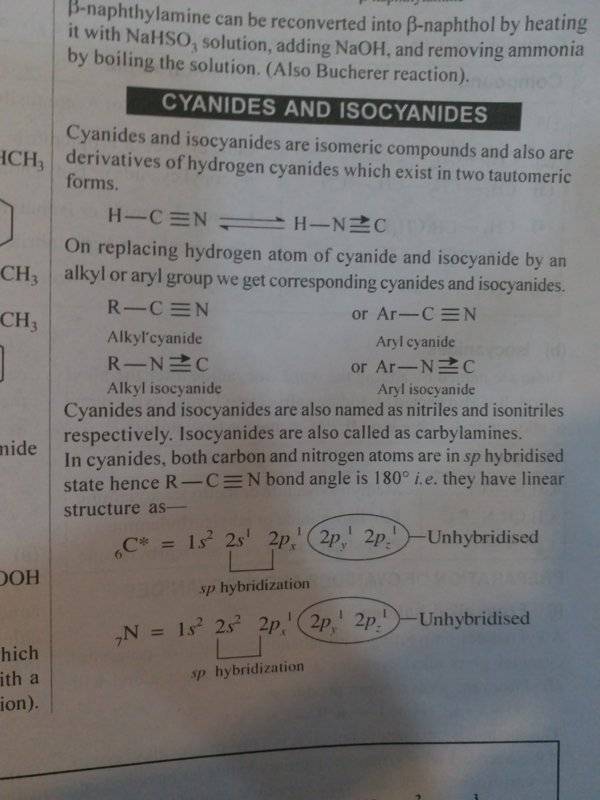
Uh is the dative structure even correct ? Or is it an error that has been persistent is a bunch of indian publications ?Sources
With Dative Bond
1)Mtg-Interactive Irganic Chemistry by Girijesh Dubey (pg 623)
2)Resonance KVPY-SX Stream : Chemistry