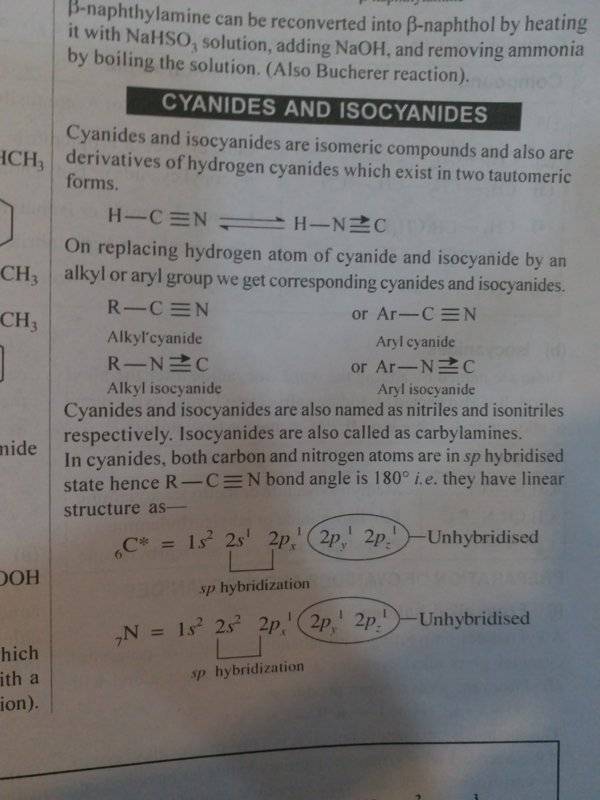Discussion Overview
The discussion revolves around the representation of isocyanides and HNC (hydrogen isocyanide) in chemical structures, specifically the use of dative (coordinate) bonds versus triple bonds with charges. Participants explore the validity and prevalence of these representations in various educational contexts, particularly in Indian publications.
Discussion Character
- Debate/contested
- Technical explanation
- Conceptual clarification
Main Points Raised
- Some participants question the correctness of representing HNC and isocyanides with a dative bond, noting that many sources do not support this structure and instead describe a triple bond with charges.
- One participant suggests that the structure can be viewed as a resonance hybrid, where nitrogen's lone pair can form a bond with an empty orbital on carbon, leading to a triple bond with formal charges.
- Another participant acknowledges that while a dative bond can be used, it is not a common representation, expressing concern about potential confusion arising from this approach.
- Some participants note that isocyanides are known to form dative bonds with metal centers, indicating a different context for the use of dative bonds.
- There is mention of other functional groups, such as the nitro group, being represented similarly with dative bonds in certain contexts, suggesting a trend in specific educational materials.
- One participant points out that the representation of ammonia-boron trifluoride can also vary, illustrating a broader pattern in how dative bonds are depicted in chemical structures.
Areas of Agreement / Disagreement
Participants express differing views on the appropriateness of using dative bonds for isocyanides and HNC, with no consensus reached on the validity of this representation. Some acknowledge its use while others find it uncommon or confusing.
Contextual Notes
Participants highlight that the representation of dative bonds may be influenced by regional educational practices, particularly in India, and that there may be a lack of clarity in how these structures are typically taught or represented in various sources.