- #1
jisbon
- 476
- 30
- Homework Statement
- 80 g solid ##SiO_{2}## is made by mixing liquid tetraethyl orthosilicate
##(Si(OC_{2}H_{5})_{4})##, ethanol ##(C_{2}H_{5}OH)##, and water at room temperature to form a
solution. The total volume of the solution is 700 ml. The ratio of the number of
water molecules to that of Si atoms is 4:1. The solution is then processed, which
involves various heat treatments, to form solid ##SiO_{2}##. The mass densities of ##SiO_{2}##,
##(Si(OC_{2}H_{5})_{4})##, ##(C_{2}H_{5}OH)## and water are 2.2 g/cm3, 0.934 g/cm3, 0.801 g/cm3 and 1g/cm3, respectively. Assume that all Si atoms have been converted to ##SiO_{2}##.
Calculate the volume of tetraethyl orthosilicate, ethanol and water in the solution (give
your answer in ml).
- Relevant Equations
- amt = mass/mr
density = mass/volume
no. of atoms = amt * ##(6.02*10^{23})##
My answer seems to be way-off/improbable, so I figured something is wrong with it.
From the periodic table,
Mr of tetraethyl orthosilicate = 208.33
Mr of ethanol = 46.069
Mr of water = 18.015
Mr of SiO2 = 60.084
Let the volume of tetraethyl orthosilicate, ethanol and water be x,y,z ml respectively.
x + y + z =700 --- (1)
Since density = mass/volume,
Mass of tetraethyl orthosilicate = 0.934x
Mass of ethanol = 0.801y
Mass of water = z
Since the mass of SiO2 is 80g, can I assume the mass of the above solutions equates to 80g?
If I can:
0.934x+0.801y+z = 80? --- (2)
Also, since ratio of water molecules to Si atoms is 4:1,
No. of Si atoms = amt of Si * (6.02*10^23)
= 0.934x/208.33 * (6.02*10^23)
= 2.69*10^21 x
Amt of water = z/18.015
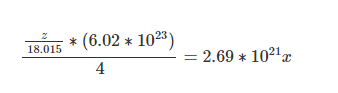
z= 0.3219x ---- (3)
Solving equations 1,2 and 3,
my values are weird and definitely incorrect (there were some negatives).
Any ideas? Cheers
From the periodic table,
Mr of tetraethyl orthosilicate = 208.33
Mr of ethanol = 46.069
Mr of water = 18.015
Mr of SiO2 = 60.084
Let the volume of tetraethyl orthosilicate, ethanol and water be x,y,z ml respectively.
x + y + z =700 --- (1)
Since density = mass/volume,
Mass of tetraethyl orthosilicate = 0.934x
Mass of ethanol = 0.801y
Mass of water = z
Since the mass of SiO2 is 80g, can I assume the mass of the above solutions equates to 80g?
If I can:
0.934x+0.801y+z = 80? --- (2)
Also, since ratio of water molecules to Si atoms is 4:1,
No. of Si atoms = amt of Si * (6.02*10^23)
= 0.934x/208.33 * (6.02*10^23)
= 2.69*10^21 x
Amt of water = z/18.015
z= 0.3219x ---- (3)
Solving equations 1,2 and 3,
my values are weird and definitely incorrect (there were some negatives).
Any ideas? Cheers