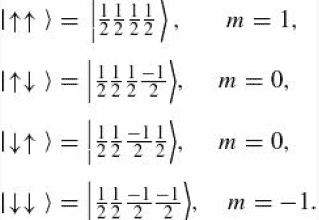Homework Help Overview
The discussion revolves around the ket notation used in quantum mechanics, specifically for representing the spin states of particles, particularly spin 1/2 particles like electrons. Participants are exploring why there are four elements in each ket notation instead of just two, as one might expect from the binary nature of spin states.
Discussion Character
- Conceptual clarification, Assumption checking
Approaches and Questions Raised
- Participants question the representation of spin states in ket notation, particularly the inclusion of four elements. Some discuss the implications of spin values and the notation used in quantum mechanics literature, referencing specific examples from Griffiths' work.
Discussion Status
There is an ongoing exploration of the notation and its implications, with some participants expressing clarity after engaging with the material. However, the discussion remains open as different interpretations of the notation are being considered.
Contextual Notes
Participants note that the notation can vary between editions of textbooks, indicating potential confusion or differing conventions in representing quantum states.