SUMMARY
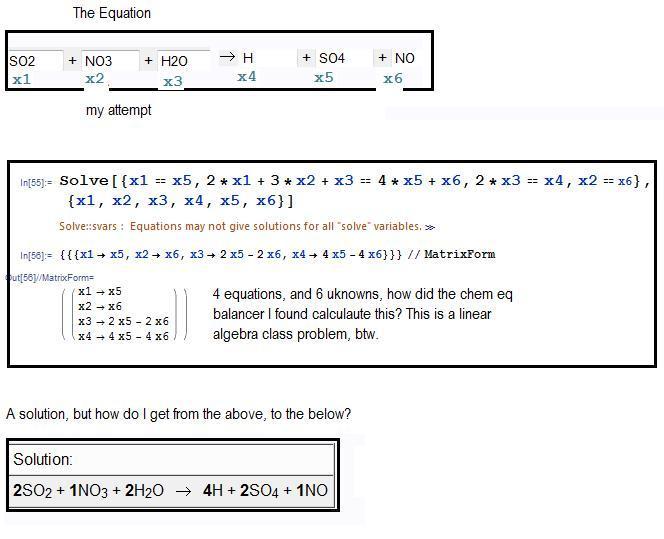
The discussion centers on balancing chemical equations in an underdetermined system, specifically with 4 equations and 6 unknowns. Jeff highlights that having fewer equations than unknowns is common and can still yield valid solutions by ensuring coefficients are integers and minimal. Borek emphasizes the importance of chemical sense in the equations, suggesting that charge balance must be maintained, which introduces additional constraints. Various solutions are presented, demonstrating different combinations of reactants and products.
PREREQUISITES
- Understanding of chemical equations and balancing techniques
- Familiarity with integer coefficients in chemical reactions
- Knowledge of charge balance in chemical species
- Experience with chemical calculators or computational chemistry tools
NEXT STEPS
- Explore methods for balancing underdetermined chemical systems
- Learn about charge balance in redox reactions
- Investigate the use of chemical calculators for complex equations
- Study integer programming techniques in chemical equation balancing
USEFUL FOR
Chemistry students, educators, and professionals involved in chemical analysis or computational chemistry who seek to enhance their understanding of balancing complex chemical equations.