- #1
JeffNYC
- 26
- 0
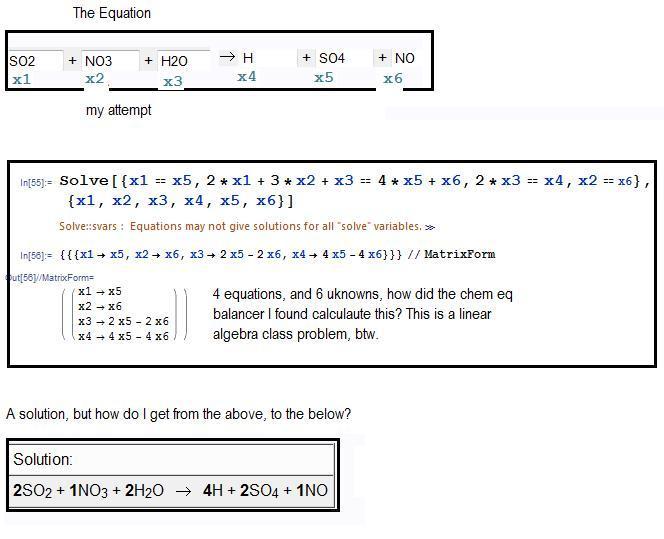
All relevant data is attached in a .jpeg.
Ultimately, I have 4 equations with 6 unknowns. I found the correct answer through a chem calculator, but I'm wondering how it computed the balanced equation.
Any insight much appreciated!
Jeff
Ultimately, I have 4 equations with 6 unknowns. I found the correct answer through a chem calculator, but I'm wondering how it computed the balanced equation.
Any insight much appreciated!
Jeff