- #1
brettlawrie
- 2
- 0
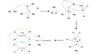
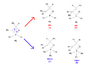
With regards to Wittig reactions, how does one know whether the cis or trans isomer is favoured? More specifically, can cis-stilbene be prepared in the attached Wittig reaction? Experimentally, the trans isomer was recovered.
I realize that trans isomers are generally more stable, but am confused as to why certain isomers are produced over their counterpart.
Thank you for your help.
I realize that trans isomers are generally more stable, but am confused as to why certain isomers are produced over their counterpart.
Thank you for your help.