KShah423
- 11
- 1
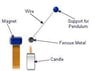
I'm doing an experiment regarding Nickel's time for a Curie point. The nickel will be connected to a wire on a pendulum and will be attracted to a neodymium magnet, but it will not be touching it. A candle will be placed in front of the magnet so that when the nickel moves toward the magnet as it is attracted to it, the fire will act as a "shield" and prevent the metal from touching the magnet. at a certain point in time, the fire will increase the nickel's temperature until it reaches the Curie point. When this occurs, the metal (Nickel) will retract from the magnet as the atoms will be shuffling around hysterically, making the metal paramagnetic. If I were to add two or more magnets, will that affect the time for the fire to increase Nickel to its Curie point? Any advice is appreciated.