ssb
- 119
- 0
While studying the structure of 1,3 butadiene and considering frontier orbital theory, I came across a graph of the HOMO to LUMO orbitals.
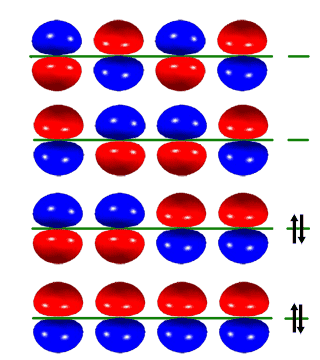
Basically put, when I draw out all the res structures for 1,3 butadiene, i can make 4 of them (i know that 2 of them are very unstable and 1 of them is very very very unstable).
Is there a similarity here or is it just dumb luck that there are 4 of each (4 res structures and 4 alignments in the homo/lumo chart.)
Thanks
Basically put, when I draw out all the res structures for 1,3 butadiene, i can make 4 of them (i know that 2 of them are very unstable and 1 of them is very very very unstable).
Is there a similarity here or is it just dumb luck that there are 4 of each (4 res structures and 4 alignments in the homo/lumo chart.)
Thanks