Waffle24
- 18
- 3
Homework Statement
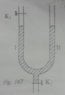
The open legs 1 and 2 of an U-shaped tube have a diameter of 2 cm^2. In leg 2, mercury is poured. When the distance is 16cm from the mercury level up to the valve K1 , then the valve gets closed. The barometer reading is 76cm Hg.
a) How big is the pressure of the sealed air?
b) Opening valve K2, then the mercury in leg 2 appears to drop faster than leg 1. Explain this.
c) When the sealed air has reached a length of 19 cm, this valve K2 is closed.
How big is the pressure of the sealed air and how much cm^3 Hg has been drained?
So I had no trouble answering question a, the answer is air pressure = sealed air.
Now comes question b. My teacher told me that the pressure in leg 1 is bigger than the pressure in leg 2, that's why the mercury in leg 2 is dropping faster, but I still fail to see how the pressure is of leg 1 is bigger than leg 2.
c) I'm kinda lost on what equation(s) I have to use to calculate the pressure and how much cm^3 Hg has been drained. It's like a few informations are missing, but then again I might be wrong and there is way out there to calculate it.
Homework Equations
So far I've got only :
- P = F/A (P = Pressure, F = Force, A = Area)
- P1 x v1 = P2 x v2 ( P= Pressure , v = Volume)
The Attempt at a Solution
N/A