- #1
xilc
- 15
- 0
I just don't get it... still... I don't get it at all... it makes no sense to me... Where can I learn how to read these ?
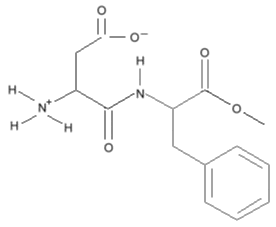
does anyone have any insight as to a guide how to read these or can you explain ? I don't get how structural formula is read...
does anyone have any insight as to a guide how to read these or can you explain ? I don't get how structural formula is read...