SUMMARY
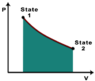
This discussion focuses on determining whether work is done on or by a closed thermodynamic system using a pV diagram. It is established that a clockwise cycle indicates positive work done by the system, while a counterclockwise cycle indicates negative work. The segments of the diagram are analyzed, revealing that the segment from 2 to 3 represents positive work, the segment from 3 to 1 represents negative work, and the segment from 1 to 2 indicates zero work due to no volume change. The area under the curve in the pV diagram directly correlates to the magnitude of work done.
PREREQUISITES
- Understanding of closed thermodynamic systems
- Familiarity with pV diagrams
- Knowledge of work-energy principles in thermodynamics
- Basic concepts of pressure and volume relationships
NEXT STEPS
- Study the principles of thermodynamic cycles
- Learn how to calculate work from area under the curve in pV diagrams
- Explore the implications of pressure changes on work done in thermodynamic systems
- Investigate the differences between positive and negative work in thermodynamic processes
USEFUL FOR
Students studying thermodynamics, educators teaching thermodynamic principles, and professionals in engineering fields focused on energy systems.