SpectraPhy09
- 23
- 3
- Homework Statement
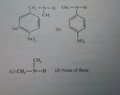
- Which of the following amine(s) will show positive Libermann's Nitroso test ?
(It is multiple choice question)
(Plz see the Options in the image I have attached)
- Relevant Equations
- I don't know any
My answer is coming a & c but given ans is a, b & c in my textbook.
Plz can someone give mechanism for this reaction?
Because my teacher only told that 2° and 1° Amines give the test.
So answer should be a & c na but it's wrong :(
Plz can someone give mechanism for this reaction?
Because my teacher only told that 2° and 1° Amines give the test.
So answer should be a & c na but it's wrong :(