Huzaifa
- 40
- 2
Homework Statement:: [...] Molecules with an extended π system such as linear polyenes and polyaromatic compounds are well described by resonance hybrids as well as by delocalized orbitals in molecular orbital theory.
Relevant Equations:: N/A
This is not a Homework question. I was reading Resonance (chemistry) from Wikipedia. I am not able to understand this sentence, "Molecules with an extended π system such as linear polyenes and polyaromatic compounds are well described by resonance hybrids as well as by delocalized orbitals in molecular orbital theory "
What is an extended π system? Given examples of linear polyenes and polyaromatic compounds of extended π system, when I searched polyaromatic, I found bee honeycomb images.
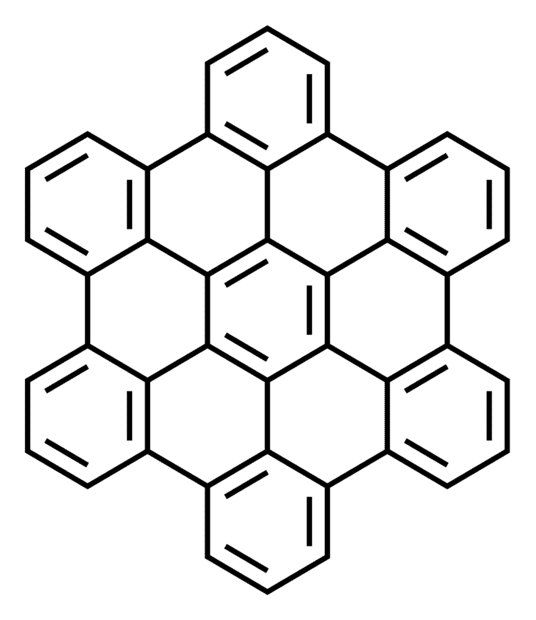
[Moderator's note: moved from a homework forum.]
Relevant Equations:: N/A
This is not a Homework question. I was reading Resonance (chemistry) from Wikipedia. I am not able to understand this sentence, "Molecules with an extended π system such as linear polyenes and polyaromatic compounds are well described by resonance hybrids as well as by delocalized orbitals in molecular orbital theory "
What is an extended π system? Given examples of linear polyenes and polyaromatic compounds of extended π system, when I searched polyaromatic, I found bee honeycomb images.
[Moderator's note: moved from a homework forum.]
Last edited by a moderator: