Discussion Overview
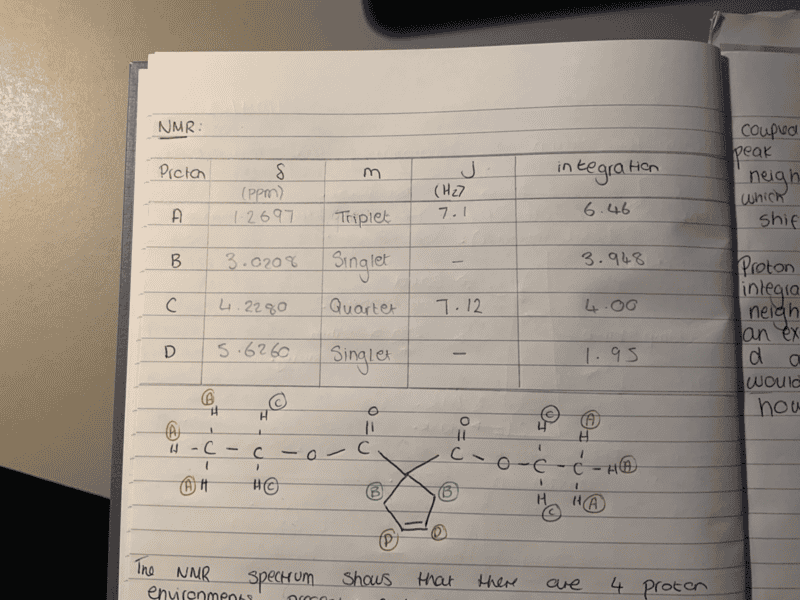
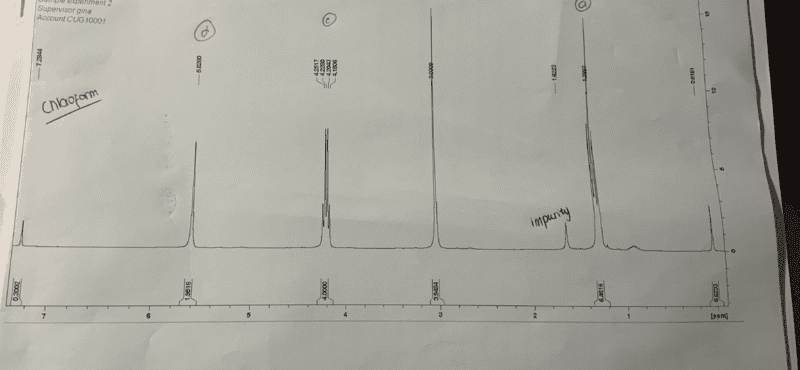
The discussion revolves around the unexpected appearance of singlets in the NMR spectrum of diethyl cyclopent-3-ene, 1, 1, dicarboxylate, where doublets and triplets were anticipated. Participants explore potential explanations for the observed multiplicity, considering both theoretical and practical aspects of NMR spectroscopy.
Discussion Character
- Exploratory
- Technical explanation
- Debate/contested
Main Points Raised
- One participant notes that the cyclic proton environments are observed as singlets instead of the expected doublets and triplets, raising questions about the reasons behind this discrepancy.
- Another participant suggests four general possibilities for the unexpected results: incorrect specimen identity, insufficient plot resolution, the need for NMR machine servicing, or incorrect expectations of results.
- A different viewpoint introduces the idea that resonance can alter the "average" observed structure, potentially affecting the spectra, although the participant expresses doubt that this applies in this case.
- One participant proposes that the orientation of the hydrogens in the ring may influence the observed multiplicity, referencing the Karplus equation and suggesting that certain orientations lead to low coupling constants.
- A participant shares a link to a proton spectrum that matches the observed results, indicating that similar spectra have been documented for the product in question.
- A question is raised regarding the impact of molecular symmetry on the observed spectrum, inviting further exploration of this aspect.
Areas of Agreement / Disagreement
Participants do not reach a consensus on the cause of the unexpected singlet appearance, with multiple competing views and hypotheses presented throughout the discussion.
Contextual Notes
Participants acknowledge various factors that could influence the NMR results, including potential issues with specimen identity, instrument resolution, and molecular orientation, but do not resolve these uncertainties.
Who May Find This Useful
This discussion may be useful for chemists and researchers interested in NMR spectroscopy, particularly those exploring molecular structure and spectral interpretation in cyclic compounds.