- #1
Raphie
- 151
- 0
aka "How to Rearrange the Elements Without Engaging in Alchemy"
Below is one view on what the Periodic Table might look like if it were "callibrated" in geometric fashion to the Atomic Numbers of the Earth Alkaline Metals. (red cells)
4, 20, 56 and 120 are all 4 times the sum of squares (1 + 4 + 9 + 16 + 25...). Multiply by 7/4 ...and you get 7, 35, 98 and 210, integers which are row sums of a Rydberg-Ritz array associated with the Hydrogen Emission Spectrum.
Subtract 2*2^2 from 20 to get 12. 2*3^2 from 56 to get 38, and 2*4^2 from 120 to get 88.
Note: The Color Coding for elements 113 - 120 are all tentative predictions. These elements have not yet been discovered. Ditto for 170 & 220.
RELEVANT ESSENTIAL INFORMATION REGARDING ALKALINE EARTH METAL ELEMENTS & HELIUM
ELEMENT---- --> SHELL CONFIGURATION --> ATOMIC NUMBER
HELIUM----- --> 02 -------------------------------> 002
BERYLLLIUM --> 02,02 ------------------------------> 04
MAGNESIUM --> 02,08,02 ----------------------------> 12
CALCIUM---- --> 02,08 08,02 -------------------------> 20
STRONTIUM --> 02,08,18,08,02 ---------------------> 38
BARIUM----- --> 02,08,18,18,08,02 ----------------> 56
RADIUM----- --> 02,08,18,32,18,08,02------------> 88
UNBINILIUM --> 02,08,18,32,32,18,08,02------> 120 (one hypothesized "Island of Stability")
Note the symmetrical structure...
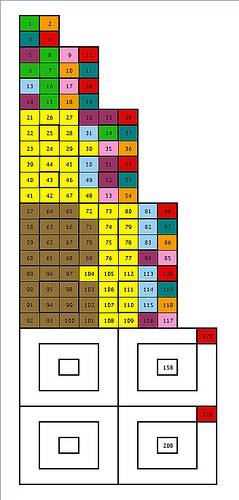
What I find particularly interesting about this is that it incorporates within it's structure the rare Earth elements.
Here is a link to the standard periodic table from Chemistry.com with atomic numbers included:
http://www.chemicalelements.com/show/atomicnumber.html"
And here is a link to a page on Wikipedia that describes other alternative versions of the periodic table: http://en.wikipedia.org/wiki/Alternative_periodic_tables"
Below is one view on what the Periodic Table might look like if it were "callibrated" in geometric fashion to the Atomic Numbers of the Earth Alkaline Metals. (red cells)
4, 20, 56 and 120 are all 4 times the sum of squares (1 + 4 + 9 + 16 + 25...). Multiply by 7/4 ...and you get 7, 35, 98 and 210, integers which are row sums of a Rydberg-Ritz array associated with the Hydrogen Emission Spectrum.
Subtract 2*2^2 from 20 to get 12. 2*3^2 from 56 to get 38, and 2*4^2 from 120 to get 88.
Note: The Color Coding for elements 113 - 120 are all tentative predictions. These elements have not yet been discovered. Ditto for 170 & 220.
RELEVANT ESSENTIAL INFORMATION REGARDING ALKALINE EARTH METAL ELEMENTS & HELIUM
ELEMENT---- --> SHELL CONFIGURATION --> ATOMIC NUMBER
HELIUM----- --> 02 -------------------------------> 002
BERYLLLIUM --> 02,02 ------------------------------> 04
MAGNESIUM --> 02,08,02 ----------------------------> 12
CALCIUM---- --> 02,08 08,02 -------------------------> 20
STRONTIUM --> 02,08,18,08,02 ---------------------> 38
BARIUM----- --> 02,08,18,18,08,02 ----------------> 56
RADIUM----- --> 02,08,18,32,18,08,02------------> 88
UNBINILIUM --> 02,08,18,32,32,18,08,02------> 120 (one hypothesized "Island of Stability")
Note the symmetrical structure...
What I find particularly interesting about this is that it incorporates within it's structure the rare Earth elements.
Here is a link to the standard periodic table from Chemistry.com with atomic numbers included:
http://www.chemicalelements.com/show/atomicnumber.html"
And here is a link to a page on Wikipedia that describes other alternative versions of the periodic table: http://en.wikipedia.org/wiki/Alternative_periodic_tables"
Last edited by a moderator: