Originaltitle
- 16
- 0
% yield questions (URGENT)
We have 3 equations:
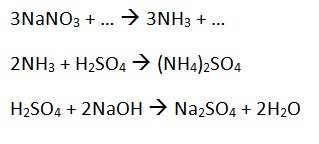
1.64 g of an impure NaNO3-containing substance is reacted with Devarda's alloy. The amount of NH3 got from this reaction is reacted with 25cm3 1.00 moldm-3 H2SO4. The H2SO4 left over is reacted with 16.2 cm3 2.00 moldm-3 NaOH. Calculate the percentage yield of NaNO3 in the impure substance.2. The attempt at a solution
My attempt at an answer:
1. Amount of H2SO4 reacted with NaOH = (2.00 x 16.2 x 10-3) / 2 = 0.0162 moles.
2. Amount of H2SO4 reacted with NH3 = 0.025 - 0.0162 = 0.0088 moles.
3. Amount of NH3 reacted = (0.0088 x 2) = 0.0176 moles.
4. Amount of NaNO3 reacted = 0.0176 x (3/2) = 0.0264.
5. Mass of NaNO3 reacted = 0.0264 x 85 = 2.244 g.
% yield = 2.244/1.64 = 137 %.
It's wrong because the final mass can't be more than the initial. HELP!
Homework Statement
We have 3 equations:
1.64 g of an impure NaNO3-containing substance is reacted with Devarda's alloy. The amount of NH3 got from this reaction is reacted with 25cm3 1.00 moldm-3 H2SO4. The H2SO4 left over is reacted with 16.2 cm3 2.00 moldm-3 NaOH. Calculate the percentage yield of NaNO3 in the impure substance.2. The attempt at a solution
My attempt at an answer:
1. Amount of H2SO4 reacted with NaOH = (2.00 x 16.2 x 10-3) / 2 = 0.0162 moles.
2. Amount of H2SO4 reacted with NH3 = 0.025 - 0.0162 = 0.0088 moles.
3. Amount of NH3 reacted = (0.0088 x 2) = 0.0176 moles.
4. Amount of NaNO3 reacted = 0.0176 x (3/2) = 0.0264.
5. Mass of NaNO3 reacted = 0.0264 x 85 = 2.244 g.
% yield = 2.244/1.64 = 137 %.
It's wrong because the final mass can't be more than the initial. HELP!