jagadeeshr
- 11
- 0
Hi,
I recently started working on MPD Thrusters. During a recent experiment, spectral images (uncalibrated) were obtained.
The thruster is made of copper and the propellant is argon. These two elements will be the source of emission spectral lines.
I need help to interpret them. I have attached spectrums and profiles.
Thank you.
Spectrum 1
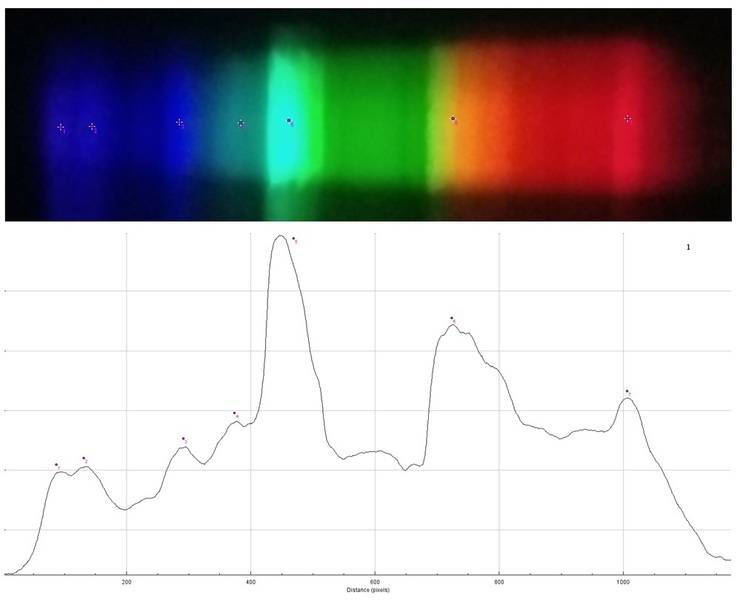
Spectrum 2
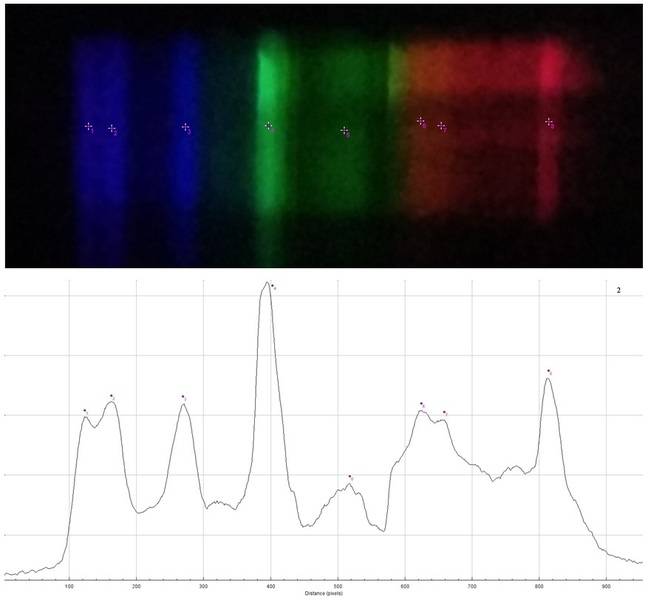
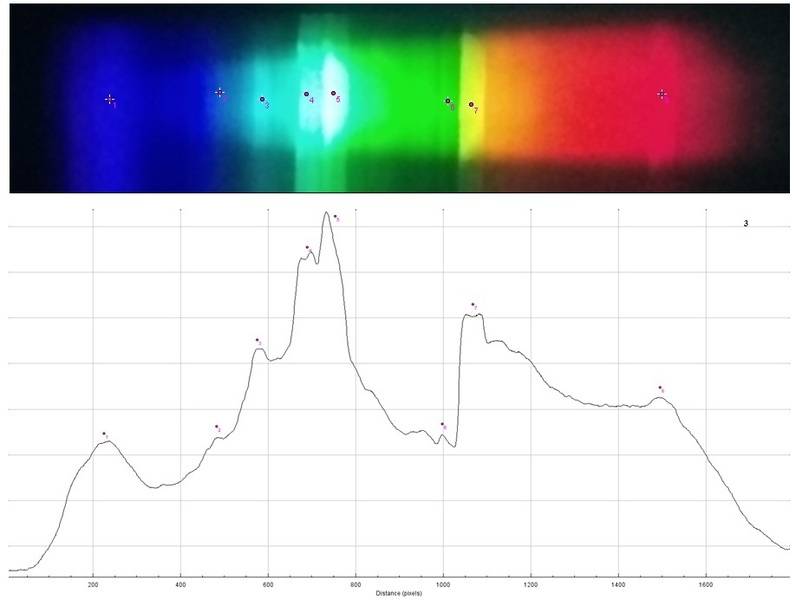
Spectrum 3
I recently started working on MPD Thrusters. During a recent experiment, spectral images (uncalibrated) were obtained.
The thruster is made of copper and the propellant is argon. These two elements will be the source of emission spectral lines.
I need help to interpret them. I have attached spectrums and profiles.
Thank you.
Spectrum 1
Spectrum 2
Spectrum 3