Coco12
- 272
- 0
1) Problem Statement
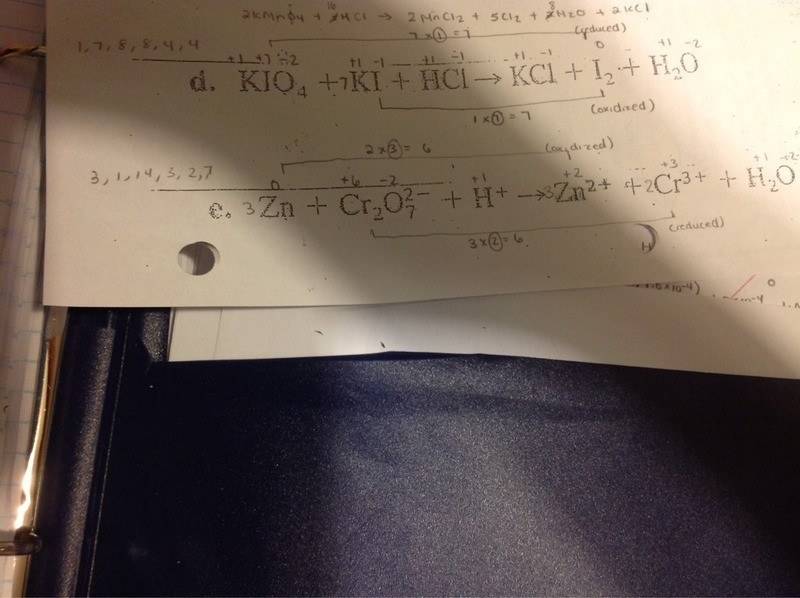 2) Relevant Equations3) Attempt at Solution
2) Relevant Equations3) Attempt at Solution
For question d, I realize in this case that the substance that is oxidized and reduced is the same molecule : iodine.
How do I proceed then as the next step would ask for you to find the change in oxidation numbers and multiplying by coefficients?
You would have 2 different changes number ( 7 and 1)?
For question d, I realize in this case that the substance that is oxidized and reduced is the same molecule : iodine.
How do I proceed then as the next step would ask for you to find the change in oxidation numbers and multiplying by coefficients?
You would have 2 different changes number ( 7 and 1)?