- #1
rwooduk
- 762
- 59
We have the formula for the mass of an atom:
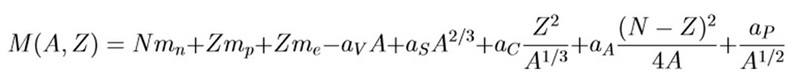
From our class notes I have:
Please could someone explain how he manages to get a single parabola from the pairing term? All I understand about the pairing term is that ap<0 for Z,N even, even. ap=0 for A odd and ap>0 for Z,N odd, odd.
At a loss if anyone can help?
From our class notes I have:
By keeping A constant and varying Z there is generally only one stable nuclide for each odd value of A. We can show this by looking at the pairing term to show that odd A gives a single parabola with a single minimum.
Please could someone explain how he manages to get a single parabola from the pairing term? All I understand about the pairing term is that ap<0 for Z,N even, even. ap=0 for A odd and ap>0 for Z,N odd, odd.
At a loss if anyone can help?