Discussion Overview
The discussion revolves around the application of Eötvös rule to calculate the surface tension of water, specifically addressing the discrepancies in results obtained by participants. The focus is on the theoretical and mathematical aspects of the calculations, including temperature measurement units and the derivation of volume from molar mass and density.
Discussion Character
- Technical explanation
- Mathematical reasoning
- Debate/contested
Main Points Raised
- One participant reports using Eötvös rule to find the surface tension of water but obtaining a value significantly lower than expected.
- Another participant emphasizes the importance of using Kelvin instead of Celsius for temperature measurements, citing the arbitrary nature of other temperature scales.
- It is noted that the equation can work in either Celsius or Kelvin, provided the same unit is used consistently.
- A participant suggests that the error may stem from incorrectly deriving volume from molar mass and density, indicating a potential oversight in the calculations.
- One participant mentions that after correcting a typo in the original post, they obtained a surface tension value closer to the expected result, but it still remains inaccurate.
- There is a reference to a Wikipedia entry suggesting a further adjustment of 6 degrees, which does not significantly improve the results.
Areas of Agreement / Disagreement
Participants express differing views on the correct approach to temperature measurement and the calculations involved. While some agree on the need for consistent units, the overall discussion remains unresolved regarding the correct application of Eötvös rule and the resulting surface tension values.
Contextual Notes
There are unresolved issues regarding the assumptions made in the calculations, particularly concerning the derivation of volume and the choice of temperature units. The discussion highlights the complexity of applying Eötvös rule accurately.
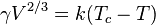 to find the surface tension of water but am not obtaining normal results.
to find the surface tension of water but am not obtaining normal results.