Franco Malgari
- 10
- 0
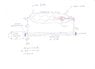
One molecule of diatomic hydrogen H2, passing through an electric arc between tungsten electrodes, splits into hydrogen atoms by absorbing a considerable amount of heat:
H2 + 103Kcal = H + H = H2 + 103Kcal
Atomic hydrogen, recombining into molecules, being unstable, after 0.5 seconds, gives a temperature of 4000 ° C.
In the literature, however, also speaks of UV lamp to operate this division and is considered a laserUV that sends its beam of light into the reactor, where it circulates hydrogen, the leaders of which there are two mirrors, one normal and one semireflecting. In this way the laser beam remains trapped inside the reactor, providing, from a certain point onwards, the energy required to trigger the cleavage of the hydrogen molecule. The right end of the reactor and then escapes atomic hydrogen H1 that, inside the heat exchanger, it recombines immediately into hydrogen H2 and providing the 4,000 ° C useful to heat the water in the circulation.
The molecular hydrogen H2 riformatosi, is then returned to the entrance of the reactor, which is thus self-sustaining.
And 'only need a bit of hydrogen H2 outside every time you turn the device.
H2 + 103Kcal = H + H = H2 + 103Kcal
Atomic hydrogen, recombining into molecules, being unstable, after 0.5 seconds, gives a temperature of 4000 ° C.
In the literature, however, also speaks of UV lamp to operate this division and is considered a laserUV that sends its beam of light into the reactor, where it circulates hydrogen, the leaders of which there are two mirrors, one normal and one semireflecting. In this way the laser beam remains trapped inside the reactor, providing, from a certain point onwards, the energy required to trigger the cleavage of the hydrogen molecule. The right end of the reactor and then escapes atomic hydrogen H1 that, inside the heat exchanger, it recombines immediately into hydrogen H2 and providing the 4,000 ° C useful to heat the water in the circulation.
The molecular hydrogen H2 riformatosi, is then returned to the entrance of the reactor, which is thus self-sustaining.
And 'only need a bit of hydrogen H2 outside every time you turn the device.