WannabeNewton said:
You need the energy eigenfunctions to form a complete set. You need to include the spherical harmonics in the radial eigenfunctions in order to make sure these eigenfunctions do form a complete set. All of this just comes from solving the 3D time-independent Schrödinger equation for the Coulomb potential. What we do is use separation of variables to break the eigenvalue equation down into a radial equation, giving us the solutions you cited, and an angular equation that gives us spherical harmonics. In the end multiply the radial solutions and the spherical harmonics together to get the complete set of solutions just like for all separation of variables problems.
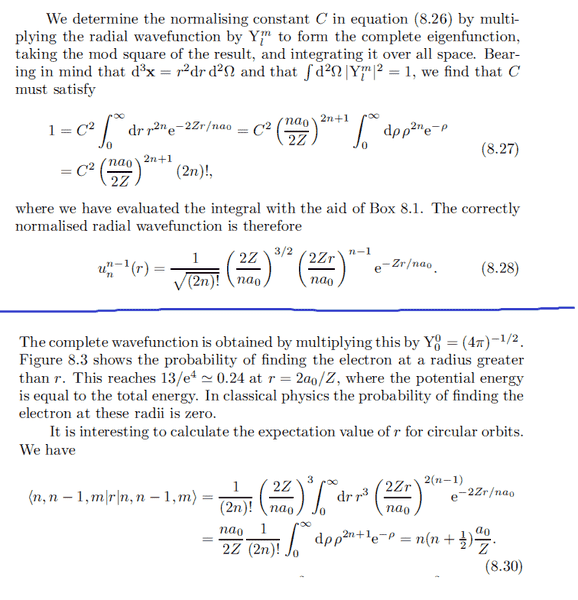
Thanks I got it. But when i try to calculate ##u_{n}^{l=n-2}##, something goes wrong:
Starting, we define operator A by:
A_{n-2} = \frac{a_0}{\sqrt 2}\left(\frac{i}{\hbar}p_r + \frac{1-n}{r} + \frac{Z}{(n-1)a_0}\right)
Substituting ##p_r = -i\hbar (\frac{\partial}{\partial r} + \frac{1}{r})##:
A_{n-2} = \frac{a_0}{\sqrt 2}\left( \frac{\partial}{\partial r} + \frac{2-n}{r} + \frac{Z}{(n-1)a_0}\right)
Thus, we want to solve:
\left(\frac{\partial}{\partial r} + \frac{2-n}{r} + \frac{Z}{(n-1)a_0} \right) u_{n}^{l=n-2} = 0
Solving by integrating factor method, we obtain:
u_n^{l=n-2} = A r^{n-2} e^{\frac{Z}{(n-1)a_0}r}
Normalizing,
u_n^{l=n-2} = \frac{1}{\sqrt{[2(n-1)]!}} \left(\frac{2Z}{(n-1)a_0}\right)^{\frac{3}{2}} \left(\frac{2Z}{(n-1)a_0}\right)^{n-2} e^{-\frac{Z}{(n-1)a_0}r}
This is similar to ##u_n^{l=n-1}##, simply replace n by n-1:
But when I substitute n = 2, so l = 0, I get ##u_2^0 = \frac{1}{\sqrt 2} \left(\frac{2Z}{a_0}\right)^{\frac{3}{2}} e^{-\frac{Z}{a_0}r}##
I get a completely different result from the book:
I'm not sure what's wrong with my derivation?