CrimpJiggler
- 141
- 1
I know that UV-Vis spectroscopy is based on the principle of electrons jumping to higher energy states but I just read that each energy state has a number of "vibrational energy levels" inside it. Heres a diagram:
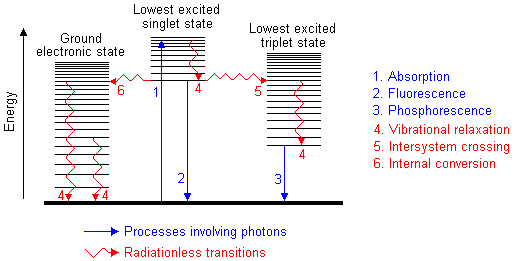
I'm confused now, what is a vibrational energy level and what is an energy state? The ground state is the state that all electrons are in before excitation and according to that diagram, the ground state has around 20 vibrational energy levels in it. Why would electrons in the ground state molecule be in any of those higher vibrational energy levels, as opposed to being in the lowest vibrational energy level?
I'm confused now, what is a vibrational energy level and what is an energy state? The ground state is the state that all electrons are in before excitation and according to that diagram, the ground state has around 20 vibrational energy levels in it. Why would electrons in the ground state molecule be in any of those higher vibrational energy levels, as opposed to being in the lowest vibrational energy level?