Discussion Overview
The discussion revolves around the interpretation of wavenumbers in Raman spectroscopy, focusing on their relation to molecular vibrations and the nature of the emitted and incident photons. Participants explore the implications of wavenumber values, the intensity of peaks in the spectrum, and the factors influencing spectral line shapes.
Discussion Character
- Exploratory
- Technical explanation
- Conceptual clarification
- Debate/contested
Main Points Raised
- Some participants question whether the wavenumber corresponds to the energy of emitted photons from deexciting molecules or the energy of the laser's photons.
- It is suggested that Raman spectroscopy involves inelastic scattering of photons, where energy conservation plays a role in determining the energy of the emitted photon.
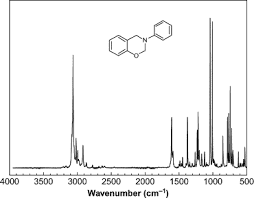
- Participants note that the horizontal axis in a Raman spectrum represents the Raman shift, which is the energy difference between incoming and scattered light, traditionally measured in cm-1.
- There is a discussion about the meaning of peaks in the spectrum, with some proposing that they correspond to changes in energy related to molecular vibrations.
- Concerns are raised about the complexity of lineshape analysis, with possibilities including low cross sections for vibrational modes or pressure broadening affecting the observed peaks.
- One participant elaborates on the process of molecular excitation and de-excitation, explaining that peaks indicate the frequency of molecular vibrations driven by incident light.
- It is mentioned that certain peaks, like those around 3000 cm-1, are typically associated with specific molecular vibrations, such as C-H vibrations.
- Factors influencing peak broadening and resolution in Raman spectra are discussed, including temperature effects and the number of vibrational modes in a molecule.
Areas of Agreement / Disagreement
Participants express various viewpoints on the interpretation of wavenumbers and the nature of the peaks in Raman spectra. There is no consensus on the exact implications of the wavenumbers or the factors affecting spectral line shapes, indicating ongoing debate and exploration of the topic.
Contextual Notes
Participants highlight the complexity of Raman spectroscopy and the nuances involved in interpreting spectral data, including the dependence on molecular structure and vibrational modes. There are also references to selection rules that determine which vibrational modes are Raman active.