Discussion Overview
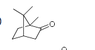
The discussion revolves around the chemical equivalence of methyl groups in a specific molecule, particularly focusing on why the top methyl groups are not considered chemically equivalent in the context of HNMR spectroscopy. The scope includes theoretical aspects of molecular structure and NMR analysis.
Discussion Character
- Homework-related, Conceptual clarification, Technical explanation
Main Points Raised
- One participant questions the chemical equivalence of the top methyl groups, suggesting they should produce only two singlets in HNMR instead of three.
- Another participant argues that the methyl groups are not equivalent due to their different positions and distances from the oxygen, as well as the inability to rotate.
- A further contribution highlights that while the distances from the oxygen differ, the primary reason for the lack of equivalence is the positioning of one methyl group within the deshielding zone of the pi system.
Areas of Agreement / Disagreement
Participants express differing views on the equivalence of the methyl groups, with some asserting they are not equivalent due to positional differences and others questioning the initial assumption of equivalence. The discussion remains unresolved regarding the exact reasons for the observed NMR signals.
Contextual Notes
Participants do not fully explore the implications of molecular geometry or the specific effects of the pi system on chemical shifts, leaving some assumptions unaddressed.