Discussion Overview
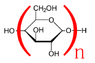
The discussion focuses on the mass change of polysaccharides, specifically starch, when linking monosaccharides like glucose. It explores the chemical process involved in polymerization, including the removal of water molecules and the resulting mass calculation.
Discussion Character
Main Points Raised
- One participant explains that the final mass of a polysaccharide is calculated as n times its empirical formula plus a water molecule, referencing the example of starch from glucose.
- Another participant clarifies that the water from each monosaccharide is already accounted for in the initial formula, suggesting that one additional water molecule should be added due to the structure of the polymer.
- A third participant emphasizes the importance of counting atoms in understanding the mass change.
Areas of Agreement / Disagreement
Participants provide differing perspectives on the mass calculation, indicating that there is no consensus on the exact reasoning behind the mass increase when linking monosaccharides into polysaccharides.
Contextual Notes
The discussion does not resolve the assumptions regarding the initial mass of the monosaccharides or the implications of the structural changes during polymerization.