Tam Le
- 23
- 1
I understand that, in the molecule H2O, O has a slight negative charge while the two H's have a slight positive charge. However, I do not understand why the molecule as a whole is considered electrically neutral.
Also, how is electrically neutral defined? Does it mean that the electrical forces of attraction and repulsion negate each other?
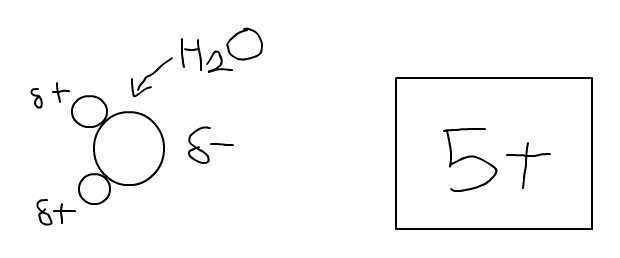
For example, in this drawing, the attraction between the O and the positively charged object would equal the repulsion between the H's and the positively charged object. Is this what being electrically neutral means?
Also, how is electrically neutral defined? Does it mean that the electrical forces of attraction and repulsion negate each other?
For example, in this drawing, the attraction between the O and the positively charged object would equal the repulsion between the H's and the positively charged object. Is this what being electrically neutral means?