Organic_Student_2020
- 1
- 0
- Homework Statement
- How to find multiplicity and J values for non symmetric 1H NMR spectra?
- Relevant Equations
- I have tried to figured this out but I can't, I need help on how to find the multiplicity of some of these arrangements of peaks. For example, there is one that looks like (from left to right), a doublet, 2 triplets, and then another doublet. I will Attach crude drawings below, but hopefully I can just get an answer on how to do this exercise.
The peaks all look unfamiliar, no symmetry, or very little, and they all integrate to a small number of hydrogen while being surrounded by a large number.
Here are the images.1.)

2.)

3.)
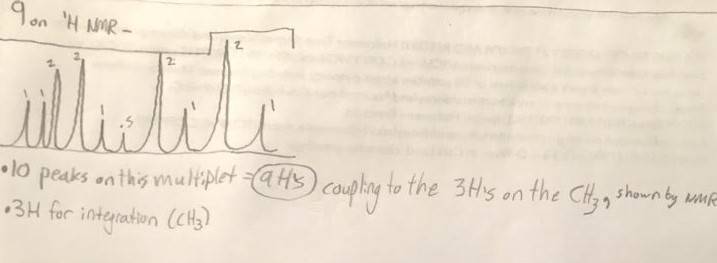
4.)

5.)

Thanks for any explanations. I don't expect anyone to do it for me, I just have no other resources to figure this out.
Here are the images.1.)
2.)
3.)
4.)
5.)
Thanks for any explanations. I don't expect anyone to do it for me, I just have no other resources to figure this out.