SUMMARY
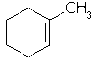
The discussion clarifies the classification of alkyl groups, specifically addressing why C2 is considered an alkyl group when attached to C1 in a double-bonded carbon structure. The CH3 group is identified as one alkyl group, while the carbon to the left of C1, referred to as C2, is also classified as an alkyl group despite its position in a double bond. The presence of the double bond does not alter the chemical identity of C1, affirming that C2 retains its classification as an alkyl group regardless of structural modifications.
PREREQUISITES
- Understanding of organic chemistry nomenclature
- Familiarity with alkyl group definitions
- Knowledge of carbon bonding and hybridization
- Basic concepts of double bonds in organic compounds
NEXT STEPS
- Research the classification of alkyl groups in organic chemistry
- Study the effects of double bonds on carbon chain structures
- Learn about the implications of structural changes in organic compounds
- Explore examples of alkyl groups in various organic molecules
USEFUL FOR
Chemistry students, organic chemists, and educators seeking to deepen their understanding of alkyl group classifications and carbon bonding structures.