Discussion Overview
The discussion revolves around the relationship between the chemical compounds S2Cl2 (disulfur dichloride) and SCl (sulfur chloride), exploring their identities, stability, and theoretical models. Participants examine the implications of their chemical formulas and the distinctions between these compounds.
Discussion Character
- Debate/contested
- Technical explanation
- Conceptual clarification
Main Points Raised
- Some participants propose that S2Cl2 and SCl are synonymous, with SCl being a simpler term, while others argue they are distinct species with different properties.
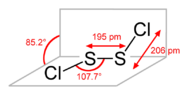
- It is noted that SCl is an unstable, short-lived species, whereas S2Cl2 is a stable liquid, leading to a preference for the term disulfur dichloride in discussions.
- One participant emphasizes that having the same empirical formula does not imply that compounds are identical, using alkenes and cycloalkanes as an analogy.
- Concerns are raised regarding the theoretical models that could support the existence of SCl, suggesting that quantum models may be necessary to account for its instability and potential dimerization.
- Another participant mentions that SCl has been studied in the gas phase, indicating its relevance despite its instability and challenges in handling it.
- Information is provided about other sulfur chloride compounds, including SCl2 and SCl4, highlighting their properties and molecular geometries.
Areas of Agreement / Disagreement
Participants do not reach a consensus on whether S2Cl2 and SCl are the same compound, with multiple competing views presented regarding their identities and properties.
Contextual Notes
The discussion reflects various assumptions about the stability and theoretical models of SCl, as well as the implications of empirical formulas in distinguishing chemical compounds.