- #1
miniradman
- 196
- 0
Homework Statement
Calculate wavelength of light the 4th line of the Balmer Series.
Homework Equations
The Rydberg Equation
The Attempt at a Solution
Do I simply sub in 2 for n1 and 6 for n2?
miniradman said:Homework Statement
Calculate wavelength of light the 4th line of the Balmer Series.
Homework Equations
The Rydberg Equation
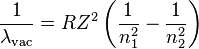
The Attempt at a Solution
Do I simply sub in 2 for n1 and 6 for n2?
The Balmer Series is a series of spectral lines in the visible region of the electromagnetic spectrum that are emitted by hydrogen atoms when electrons transition from higher energy levels to the second energy level. These lines are named after Johann Balmer, who first observed them in the 19th century.
The formula for calculating the wavelength of the 4th line of the Balmer Series is:λ = (4/3) * λ0Where λ is the wavelength of the 4th line and λ0 is the wavelength of the first line in the series (known as the Balmer limit).
The Balmer Series is significant because it provided evidence for the existence of energy levels in atoms. This discovery helped to lay the foundation for the development of quantum mechanics and our understanding of atomic structure. The series is also useful in identifying and studying hydrogen-rich objects in space, such as stars and nebulae.
The main factor that affects the Balmer Series is the energy levels of the electrons within the hydrogen atom. The higher the energy level, the longer the wavelength of the emitted light. Additionally, external factors such as temperature and pressure can also affect the spectral lines in the series.
Yes, there are several other series of spectral lines in the electromagnetic spectrum that are similar to the Balmer Series. These include the Lyman Series, which corresponds to transitions to the first energy level, and the Paschen, Brackett, and Pfund Series, which correspond to transitions to higher energy levels. These series are all named after the scientists who first observed them.