Geospinelsulfid
- 1
- 0
Hey Guys,
i am currently trying to implement the technology of this paper (https://www.cell.com/joule/fulltext/S2542-4351(23)00360-4)(Extreme salt-resisting multistage solar distillation with thermohaline convection) into a product for my Design Diploma.
As the picture hopefully helps to explain, this object gets lowered into sea water and by that gets automatically filled with it because of its various openings (gravity feeding).
Now the desalination in the main chamber can start to begin and a salinity gradient is introduced. In the chamber we have higher salinity water which is denser than the lower salinity water outside of the chamber. The Authors describe that now through the differences in salinity and density a completely passive exchange starts to happen between the high and low area so that "fresh" low salinity water always keeps flowing in and the denser high salinity water flows out.(there are two openings(not like in the image) so one might be for "in" and one for "out"
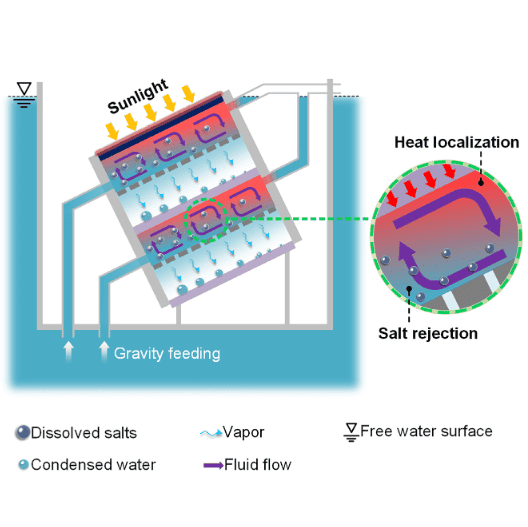
So my questions is:
Do you think this passive exchange can still happen if i close the upper opening after the object was lowered into the water?
the idea behind it would be to build a system in which this system could sit besides-behind and over each other multiple times, but a difference in height seems difficult to do because my guess would be that the efficiency worsens the more and longer the atmospheric connection is( like the second cell in the picture)because more water would be heated for nothing outside the desalination chamber. But if can open some valve to fill it once and then close that valve for good that would make my life way easier.
Feel free to ask questions or correct me anywhere because this is waay out of my knowledge base.
Happy for any help or hint thank you :)
i am currently trying to implement the technology of this paper (https://www.cell.com/joule/fulltext/S2542-4351(23)00360-4)(Extreme salt-resisting multistage solar distillation with thermohaline convection) into a product for my Design Diploma.
As the picture hopefully helps to explain, this object gets lowered into sea water and by that gets automatically filled with it because of its various openings (gravity feeding).
Now the desalination in the main chamber can start to begin and a salinity gradient is introduced. In the chamber we have higher salinity water which is denser than the lower salinity water outside of the chamber. The Authors describe that now through the differences in salinity and density a completely passive exchange starts to happen between the high and low area so that "fresh" low salinity water always keeps flowing in and the denser high salinity water flows out.(there are two openings(not like in the image) so one might be for "in" and one for "out"
So my questions is:
Do you think this passive exchange can still happen if i close the upper opening after the object was lowered into the water?
the idea behind it would be to build a system in which this system could sit besides-behind and over each other multiple times, but a difference in height seems difficult to do because my guess would be that the efficiency worsens the more and longer the atmospheric connection is( like the second cell in the picture)because more water would be heated for nothing outside the desalination chamber. But if can open some valve to fill it once and then close that valve for good that would make my life way easier.
Feel free to ask questions or correct me anywhere because this is waay out of my knowledge base.
Happy for any help or hint thank you :)