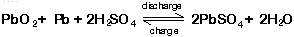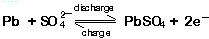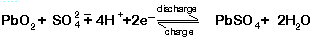Discussion Overview
The discussion revolves around the feasibility of converting old lead-acid batteries into pure lead through reverse polarity charging and other methods. Participants explore the chemical reactions involved, the composition of lead in batteries, and various reprocessing techniques.
Discussion Character
- Exploratory
- Technical explanation
- Debate/contested
Main Points Raised
- One participant questions whether reverse polarity charging after removing the liquid from old batteries could yield pure lead, referencing standard chemical reactions.
- Another participant argues that real batteries involve complex reactions and that the lead is typically alloyed with other metals, which complicates the process.
- A different viewpoint suggests that reprocessing could involve removing electrodes, washing them, and smelting, although this may not yield pure lead due to residual alloying elements.
- One participant challenges the previous claim, suggesting that smelting could produce a higher percentage of PbSO4 than anticipated, indicating a need for better recovery methods.
- Another participant proposes a method to convert PbSO4 into lead during smelting by adding charcoal and sodium carbonate, detailing the chemical reactions involved and emphasizing the importance of controlling the carbon content.
Areas of Agreement / Disagreement
Participants express differing views on the effectiveness of reverse polarity charging and smelting methods, with no consensus reached on the best approach to recover pure lead from old batteries.
Contextual Notes
Participants note the complexity of battery chemistry, the presence of alloying elements, and the potential for varying yields of lead and lead sulfate during reprocessing, highlighting the need for careful consideration of chemical reactions and material properties.