SUMMARY
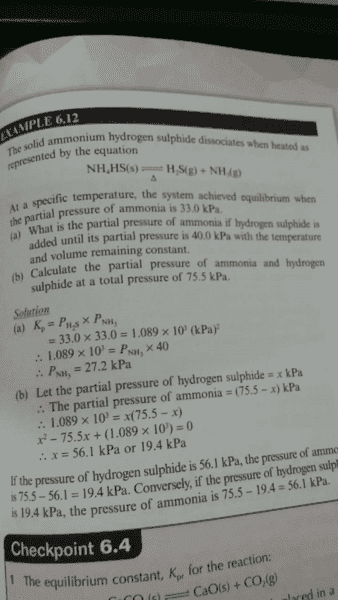
The discussion centers on the relationship between the partial pressures of H2S and NH3 in a chemical equilibrium scenario. It is established that prior to the addition of H2S, the partial pressures of H2S and NH3 are equal due to the stoichiometry of the dissociation reaction. This equality is crucial for understanding how solution (a) recognizes the equivalence of PH2S and ammonia. The participants confirm the importance of stoichiometric principles in determining equilibrium states.
PREREQUISITES
- Understanding of chemical equilibrium principles
- Knowledge of stoichiometry in chemical reactions
- Familiarity with partial pressure concepts
- Basic chemistry background, particularly in gas-phase reactions
NEXT STEPS
- Study the principles of chemical equilibrium in detail
- Explore stoichiometric calculations in gas-phase reactions
- Research the concept of partial pressures in equilibrium systems
- Examine case studies involving H2S and NH3 in chemical reactions
USEFUL FOR
Chemistry students, educators, and professionals involved in chemical research or industrial applications related to gas-phase equilibria.